Kluczowe dokumenty
909262
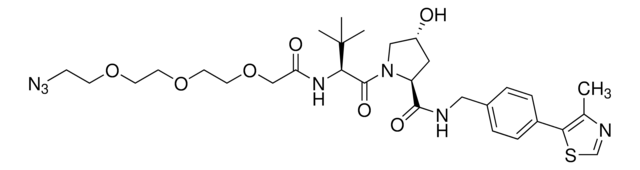
(S,R,S)-AHPC-PEG4-Azide
≥95%
Synonim(y):
(2S,4R)-1-((S)-17-Azido-2-(tert-butyl)-4-oxo-6,9,12,15-tetraoxa-3-azaheptadecanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide, Crosslinker–E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
About This Item
Polecane produkty
ligand
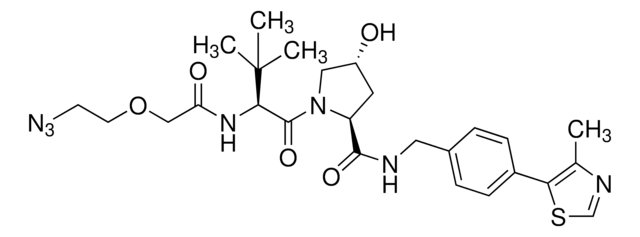
VH032
Próba
≥95%
Formularz
liquid
przydatność reakcji
reaction type: click chemistry
reagent type: ligand-linker conjugate
grupa funkcyjna
azide
temp. przechowywania
2-8°C
ciąg SMILES
O=C(COCCOCCOCCOCCN=[N+]=[N-])N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C
Zastosowanie
Inne uwagi
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of Target Warhead and Linkage Vector on Inducing Protein Degradation: Comparison of Bromodomain and Extra-Terminal (BET) Degraders Derived from Triazolodiazepine (JQ1) and Tetrahydroquinoline (I-BET726) BET Inhibitor Scaffolds
Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4
Informacje prawne
produkt powiązany
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Produkty
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej