909262
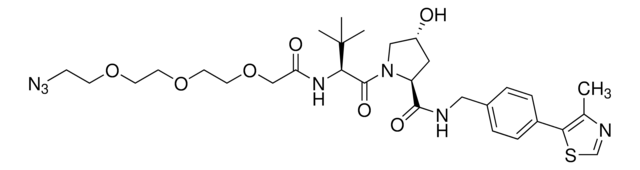
(S,R,S)-AHPC-PEG4-Azide
≥95%
Sinônimo(s):
(2S,4R)-1-((S)-17-Azido-2-(tert-butyl)-4-oxo-6,9,12,15-tetraoxa-3-azaheptadecanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide, Crosslinker–E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
About This Item
Produtos recomendados
ligand
VH032
Ensaio
≥95%
Formulário
liquid
adequação da reação
reaction type: click chemistry
reagent type: ligand-linker conjugate
grupo funcional
azide
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
O=C(COCCOCCOCCOCCN=[N+]=[N-])N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C
Aplicação
Outras notas
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of Target Warhead and Linkage Vector on Inducing Protein Degradation: Comparison of Bromodomain and Extra-Terminal (BET) Degraders Derived from Triazolodiazepine (JQ1) and Tetrahydroquinoline (I-BET726) BET Inhibitor Scaffolds
Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4
Informações legais
produto relacionado
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica