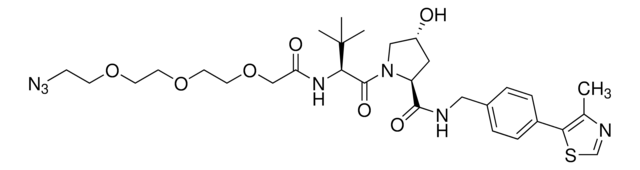
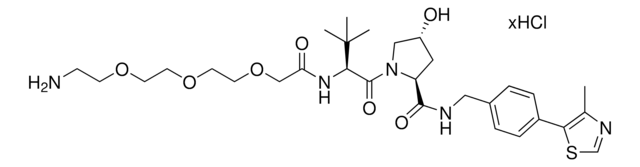
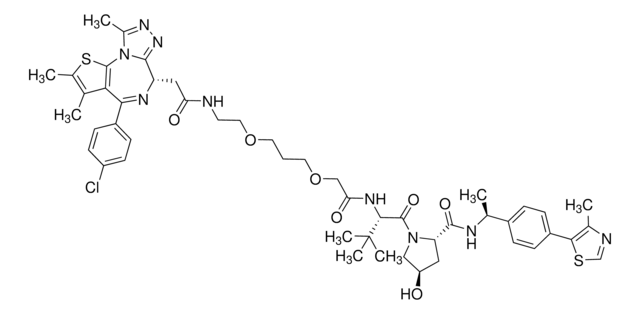
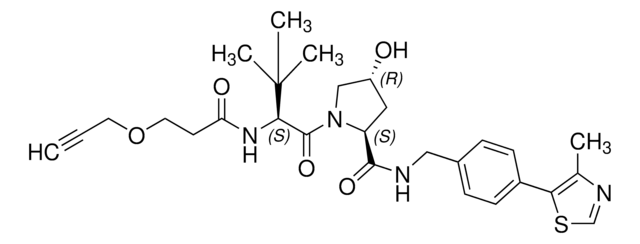
901490
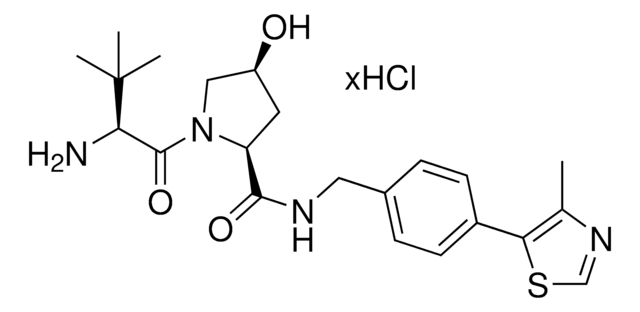
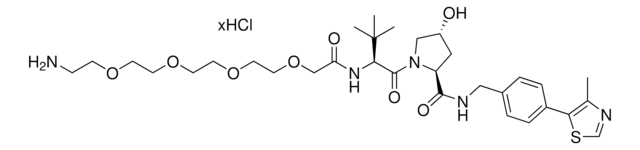
(S,R,S)-AHPC hydrochloride
≥97%
別名:
(2S,4R)-1-((S)-2-Amino-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide hydrochloride, E3 Ligase ligand, Ligand for PROTAC® research, VH032
About This Item
おすすめの製品
ligand
VH032
品質水準
アッセイ
≥97%
フォーム
powder or crystals
反応適合性
reagent type: ligand
保管温度
2-8°C
SMILES記法
N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C.Cl
InChI Key
JYRTWGCWUBURGU-MSSRUXLCSA-N
関連するカテゴリー
アプリケーション
その他情報
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Structure-Guided Design and Optimization of Small Molecules Targeting the Protein-Protein Interaction between the von Hippel-Lindau (VHL) E3 Ubiquitin Ligase and the Hypoxia Inducible Factor (HIF) Alpha Subunit with in Vitro Nanomolar Affinities
Modular PROTAC Design for the DegradationofOncogenic BCR-ABL
Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4
Group-Based Optimization of Potent and Cell-Active Inhibitors of the von Hippel–Lindau (VHL) E3 Ubiquitin Ligase: Structure–Activity Relationships Leading to the Chemical Probe (2S,4R)-1-((S)-2-(1-Cyanocyclopropanecarboxamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (VH298)
法的情報
関連製品
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
901490-VAR:
901490-BULK:
901490-100MG:
901490-BULK-KC:
901490-50MG-KC:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
資料
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)