おすすめの製品
ligand
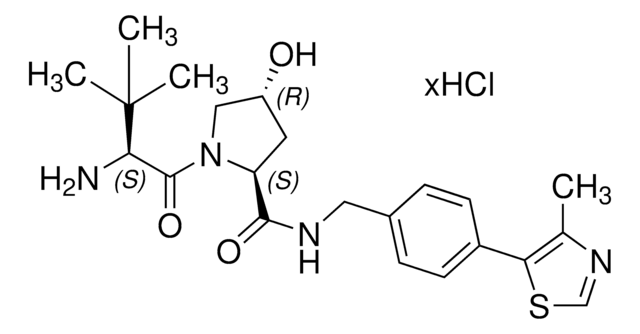
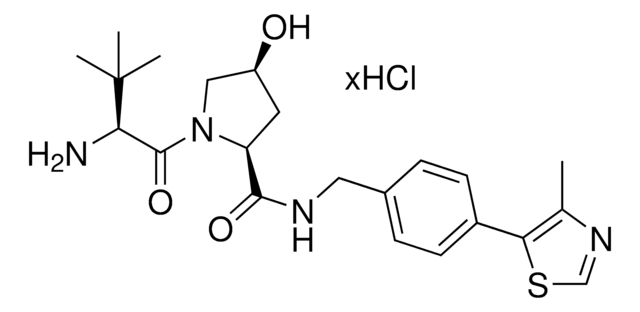
VL285 phenol
品質水準
フォーム
solid
反応適合性
reagent type: ligand
官能基
amine
保管温度
2-8°C
SMILES記法
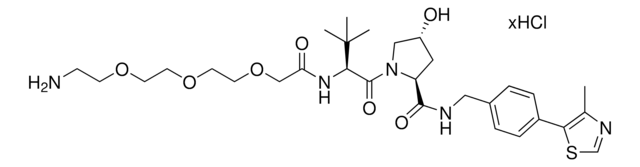
O=C([C@@H]1C[C@@H](O)CN1C([C@H](C(C)C)N2CC(C=CC=C3)=C3C2=O)=O)NCC4=CC=C(C5=C(C)N=CS5)C=C4O
InChI
1S/C29H32N4O5S/c1-16(2)25(33-13-20-6-4-5-7-22(20)28(33)37)29(38)32-14-21(34)11-23(32)27(36)30-12-19-9-8-18(10-24(19)35)26-17(3)31-15-39-26/h4-10,15-16,21,23,25,34-35H,11-14H2,1-3H3,(H,30,36)/t21-,23+,25+/m1/s1
InChI Key
KNZQRBRUYSXXRG-VTZPFEBOSA-N
関連するカテゴリー
アプリケーション
(S,R,S)-VL285 Phenol is a ligand used in the recruitment of the von Hippel-Lindau (VHL) protein for targeted protein degradation and PROTAC (proteolysis-targeting chimeras) technology, providing an alternative to the widely used VH032 (901490).
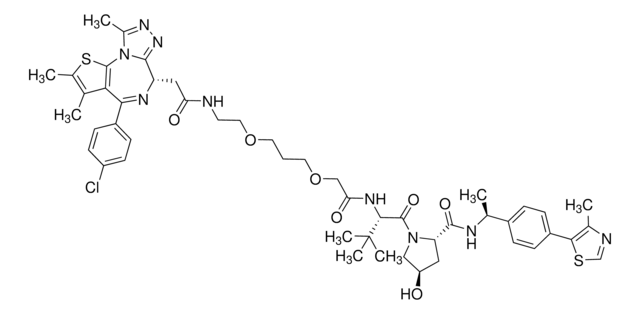
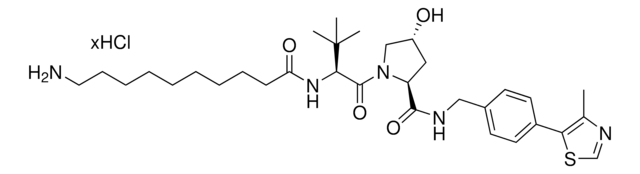
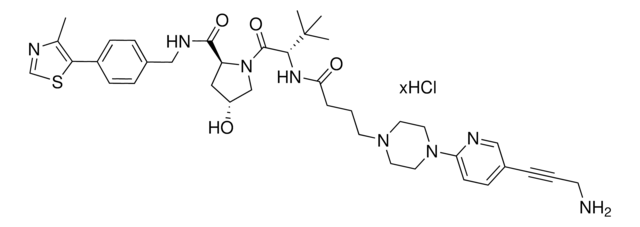
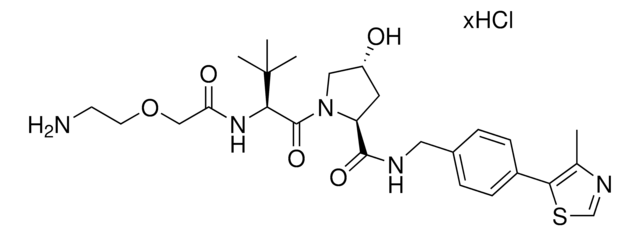
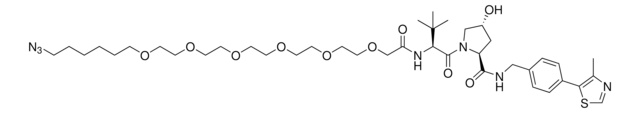
(S,R,S)-VL285 Phenol-linker conjugates are also available for synthesis of degraders. Browse our full offering of degrader building blocks that streamlines the synthesis of degrader libraries.
(S,R,S)-VL285 Phenol-linker conjugates are also available for synthesis of degraders. Browse our full offering of degrader building blocks that streamlines the synthesis of degrader libraries.
その他情報
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
Systematic exploration of different E3 ubiquitin ligases: an approach towards potent and selective CDK6 degraders
Design, synthesis and biological evaluation of Proteolysis Targeting Chimeras (PROTACs) as a BTK degraders with improved pharmacokinetic properties
Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
Systematic exploration of different E3 ubiquitin ligases: an approach towards potent and selective CDK6 degraders
Design, synthesis and biological evaluation of Proteolysis Targeting Chimeras (PROTACs) as a BTK degraders with improved pharmacokinetic properties
Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase
法的情報
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
920908-100MG:
920908-VAR:
920908-BULK:
最新バージョンのいずれかを選択してください:
Saul Jaime-Figueroa et al.
Bioorganic & medicinal chemistry letters, 30(3), 126877-126877 (2019-12-28)
A new series of Proteolysis Targeting Chimeras (PROTACs) targeting Bruton's Tyrosine Kinase (BTK) was synthesized, with the goal of improving the pharmacokinetic properties of our previously reported PROTAC, MT802. We recently described the ability of MT802 to induce degradation of
Dennis L Buckley et al.
ACS chemical biology, 10(8), 1831-1837 (2015-06-13)
Small molecule-induced protein degradation is an attractive strategy for the development of chemical probes. One method for inducing targeted protein degradation involves the use of PROTACs, heterobifunctional molecules that can recruit specific E3 ligases to a desired protein of interest.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)