901523
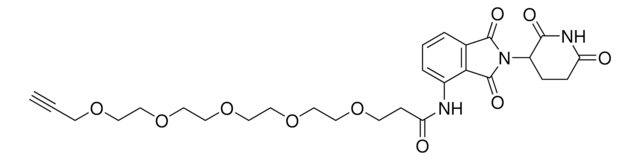
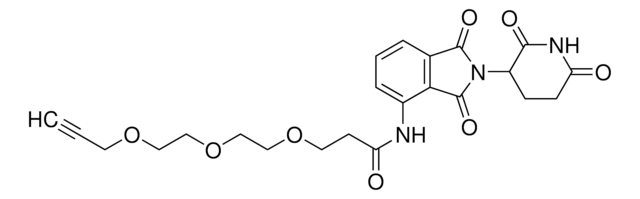
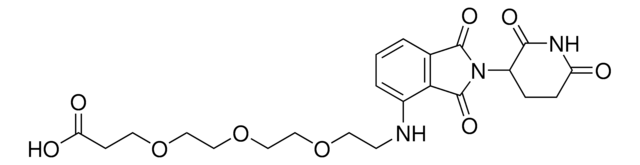
Pomalidomide-PEG1-Alkyne
≥98%
別名:
N-(2-(2,6-Dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)-3-(prop-2-yn-1-yloxy)propanamide, Crosslinker−E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
おすすめの製品
ligand
pomalidomide
品質水準
アッセイ
≥98%
フォーム
powder or crystals
反応適合性
reaction type: click chemistry
reagent type: ligand-linker conjugate
官能基
alkyne
保管温度
2-8°C
SMILES記法
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(CCOCC#C)=O)=O)NC1=O
InChI
1S/C19H17N3O6/c1-2-9-28-10-8-15(24)20-12-5-3-4-11-16(12)19(27)22(18(11)26)13-6-7-14(23)21-17(13)25/h1,3-5,13H,6-10H2,(H,20,24)(H,21,23,25)
InChI Key
NECGAXFLPKIXPG-UHFFFAOYSA-N
アプリケーション
Automate your CRBN-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
その他情報
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
法的情報
関連製品
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
901523-BULK:
901523-VAR:
901523-50MG:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
資料
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)