905224
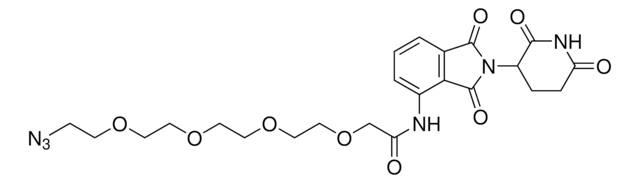
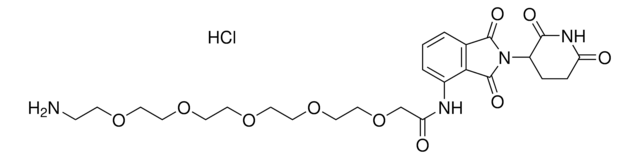
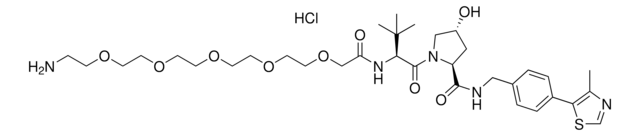
Pomalidomide-PEG6-butyl amine hydrochloride
≥95%
Sinonimo/i:
24-Amino-N-(2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin, Crosslinker–E3 Ligase ligand conjugate, Pomalidomide-2-2-2-2-2-2-6-NH2 HCl salt, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
Prodotti consigliati
ligand
pomalidomide
Saggio
≥95%
Forma fisica
powder or crystals
Impiego in reazioni chimiche
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
Gruppo funzionale
amine
Temperatura di conservazione
2-8°C
Stringa SMILE
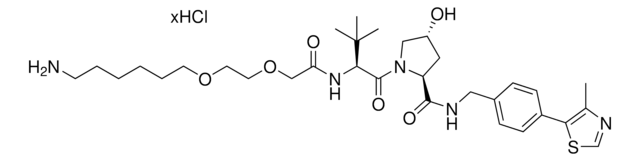
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(COCCOCCOCCOCCOCCOCCCCCCN)=O)=O)NC1=O.Cl
Applicazioni
Altre note
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Note legali
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.