Kluczowe dokumenty
B8959
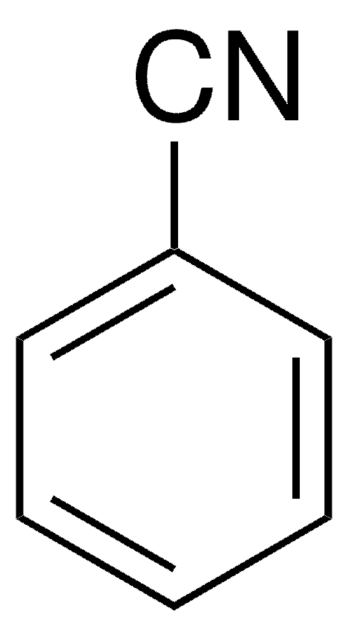
Benzonitrile
ReagentPlus®, 99%
Synonim(y):
Phenyl cyanide
About This Item
Polecane produkty
Poziom jakości
linia produktu
ReagentPlus®
Próba
99%
Formularz
liquid
granice wybuchowości
0.34-6.3 %
dilution
(for general lab use)
współczynnik refrakcji
n20/D 1.528 (lit.)
bp
191 °C (lit.)
mp
−13 °C (lit.)
ciąg SMILES
N#Cc1ccccc1
InChI
1S/C7H5N/c8-6-7-4-2-1-3-5-7/h1-5H
Klucz InChI
JFDZBHWFFUWGJE-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
- Rozpuszczalnik elektrochemiczny do badania elektrochemii, właściwości spektroskopowych i reaktywności serii porfiryn kobaltowych z różnymi podstawnikami.
- Blok konstrukcyjny lub materiał wyjściowy w różnych reakcjach syntezy organicznej.
- Stosowany w reakcjach sprzęgania, takich jak sprzęganie Suzuki lub reakcje Hecka, w celu ułatwienia tworzenia wiązań węgiel-węgiel.
Informacje prawne
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Kod klasy składowania
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
158.0 °F - closed cup
Temperatura zapłonu (°C)
70 °C - closed cup
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej