900814
SLAP TM
≥95%
Sinônimo(s):
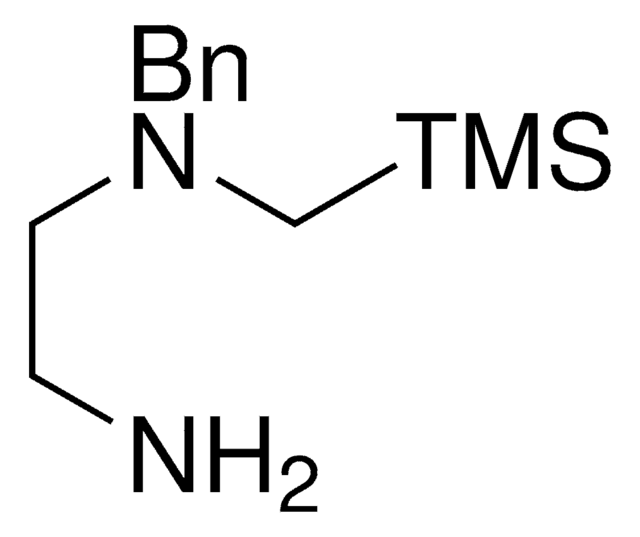
2-(((Trimethylsilyl)methyl)thio)ethanamine
About This Item
Produtos recomendados
Ensaio
≥95%
forma
liquid
índice de refração
n/D 1.4811
densidade
0.90746 g/mL
grupo funcional
amine
thioether
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
NCCSC[Si](C)(C)C
Aplicação
Outras notas
- Technology Spotlight: SLAP Reagents for Piperazine Synthesis
- Silicon Amine Reagents for the Photocatalytic Synthesis of Piperazines from Aldehydes and Ketones
- Lewis Acid Induced Toggle from Ir(II) to Ir(IV) Pathways in Photocatalytic Reactions: Synthesis of Thiomorpholines and Thiazepanes from Aldehydes and SLAP Reagents.
- Continuous Flow Synthesis of Morpholines and Oxazepanes with Silicon Amine Protocol (SLAP) Reagents and Lewis Acid Facilitated Photoredox Catalysis
produto relacionado
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
174.0 °F
Ponto de fulgor (°C)
78.89 °C
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Protocolos
The Bode group has developed SnAP (stannyl amine protocol) reagents that cross-couple with aldehydes and ketones to provide one-step access to a wide variety of saturated N-heterocycles.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica