798878
SnAP M Reagent
95%
Sinônimo(s):
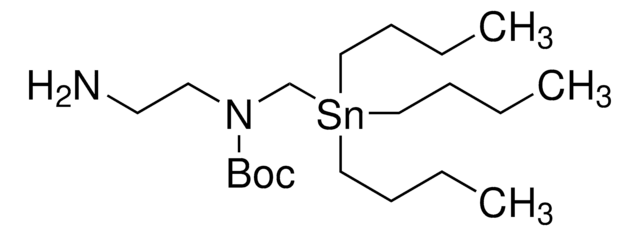
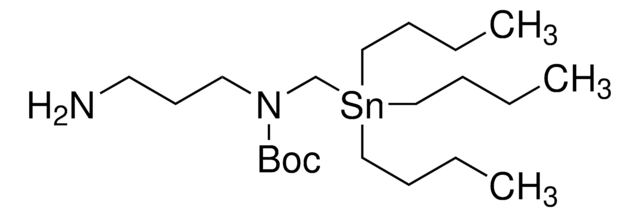
2-[(tributylstannyl)methoxy]-Ethanamine
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
95%
forma
liquid
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
CCCC[Sn](CCCC)(COCCN)CCCC
InChI
1S/3C4H9.C3H8NO.Sn/c3*1-3-4-2;1-5-3-2-4;/h3*1,3-4H2,2H3;1-4H2;
chave InChI
YFZRXKFYLGAQOC-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
Automate your N-heterocycle formation with Synple Automated Synthesis Platform (SYNPLE-SC002)
Outras notas
Professor product portal: Jeffrey Bode Research Group
SnAP Reagents for the Synthesis of Piperazines and Morpholines
SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes
SnAP Reagents for a Cross-Coupling Approach to the One-Step Synthesis of Saturated N-Heterocycles
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
Saturated N-heterocyclic building blocks or SnAP Reagents are of growing importance for the convenient synthesis of medium-ring saturated N-heterocycles, including bicyclic and spirocyclic structures. SnAP reagents are stable and readily available and can be coupled with widely available aromatic, heteroaromatic, aliphatic, and glyoxylic aldehydes.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica