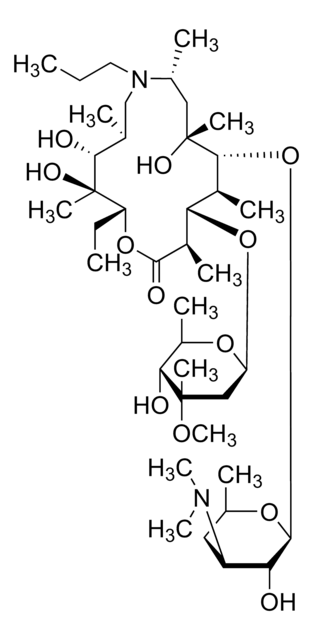
V2753
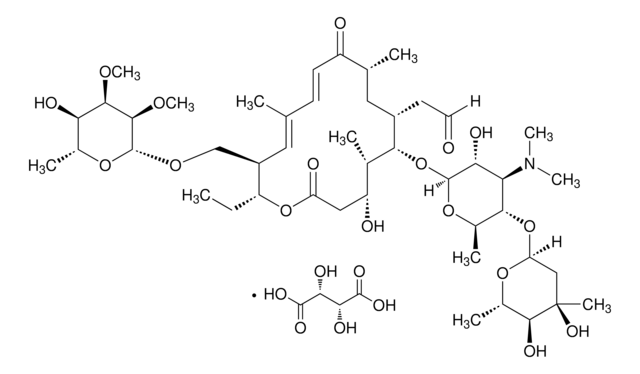
Virginiamycin M1
≥95% (HPLC)
Sinônimo(s):
Ostreogrycin A, Pristinamycin IIA, Staphylomycin M1, Streptogramin A, Virginiamycin M1, Mikamycin A, Staphylomycin
About This Item
Produtos recomendados
fonte biológica
Streptomyces virginiae
Nível de qualidade
Ensaio
≥95% (HPLC)
forma
powder
condição de armazenamento
(Keep container tightly closed in a dry and well-ventilated place.)
cor
white to yellow
solubilidade
methanol: soluble 10 mg/mL
espectro de atividade do antibiótico
Gram-positive bacteria
Modo de ação
protein synthesis | interferes
temperatura de armazenamento
2-8°C
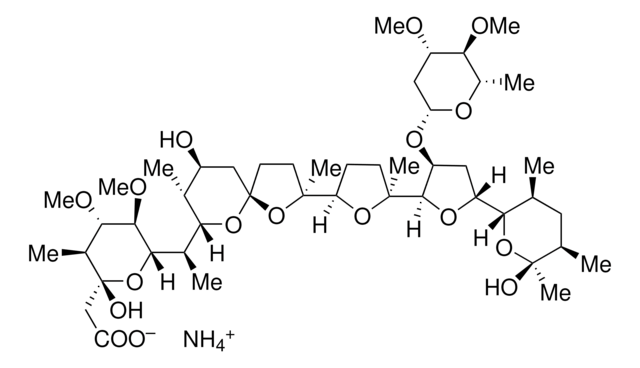
cadeia de caracteres SMILES
CC(C)[C@H]1OC(=O)C2=CCCN2C(=O)c3coc(CC(=O)C[C@H](O)C=C(C)C=CCNC(=O)C=C[C@H]1C)n3
InChI
1S/C28H35N3O7/c1-17(2)26-19(4)9-10-24(34)29-11-5-7-18(3)13-20(32)14-21(33)15-25-30-22(16-37-25)27(35)31-12-6-8-23(31)28(36)38-26/h5,7-10,13,16-17,19-20,26,32H,6,11-12,14-15H2,1-4H3,(H,29,34)/b7-5-,10-9-,18-13-/t19-,20-,26-/m1/s1
chave InChI
DAIKHDNSXMZDCU-QHKJSJJMSA-N
Categorias relacionadas
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
Outras notas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica