L5768
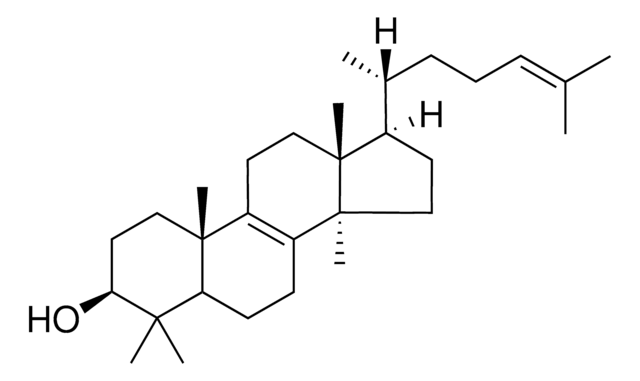
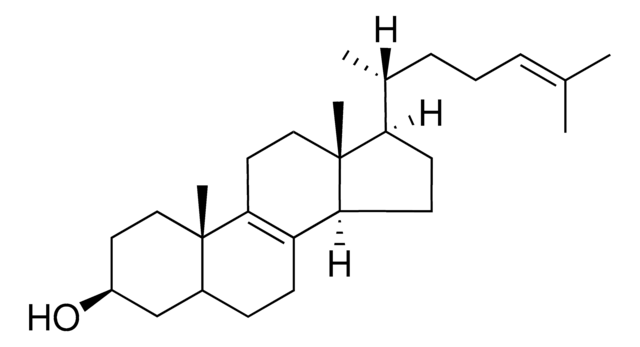
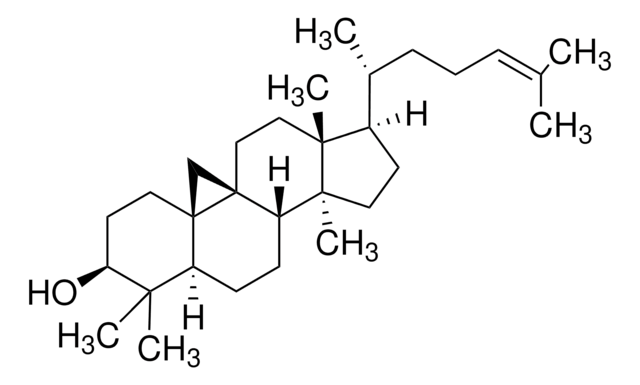
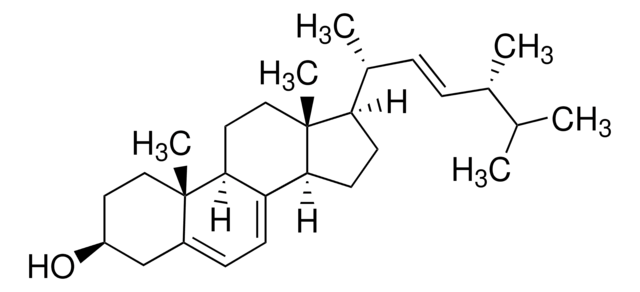
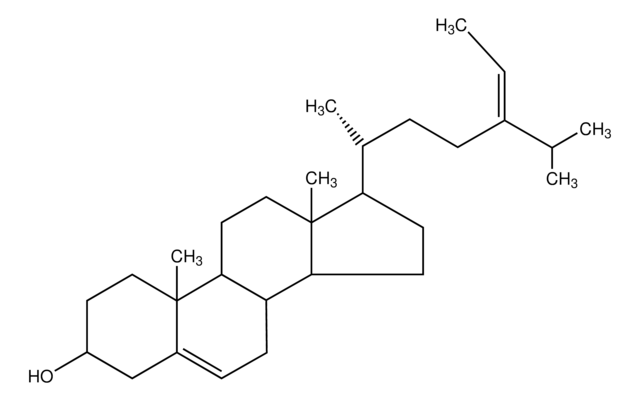
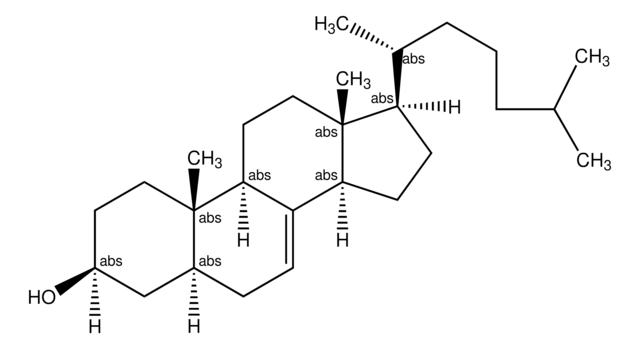
Lanosterol
≥93%, powder
Sinônimo(s):
3β-Hydroxy-8,24-lanostadiene, 8,24-Lanostadien-3β-ol
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥93%
Formulário
powder
cor
white to off-white
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
[H][C@@]1(CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]4(C)CC[C@H](O)C(C)(C)[C@]4([H])CC3)[C@H](C)CC\C=C(/C)C
InChI
1S/C30H50O/c1-20(2)10-9-11-21(3)22-14-18-30(8)24-12-13-25-27(4,5)26(31)16-17-28(25,6)23(24)15-19-29(22,30)7/h10,21-22,25-26,31H,9,11-19H2,1-8H3/t21-,22-,25+,26+,28-,29-,30+/m1/s1
chave InChI
CAHGCLMLTWQZNJ-BQNIITSRSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- as a standard in HPLC for the quantification in testis samples
- in S-adenosyl-L-methionine:Δ24-sterol-C-methyltransferase (SMT) assay
- to treat wild-type cells growing in rich medium to know its effects on Sre1 protein
Ações bioquímicas/fisiológicas
Lanosterol serves as an endogenous selective modulator of macrophage immunity.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
Conteúdo relacionado
Discover Bioactive Small Molecules for Lipid Signaling Research
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica