A7410
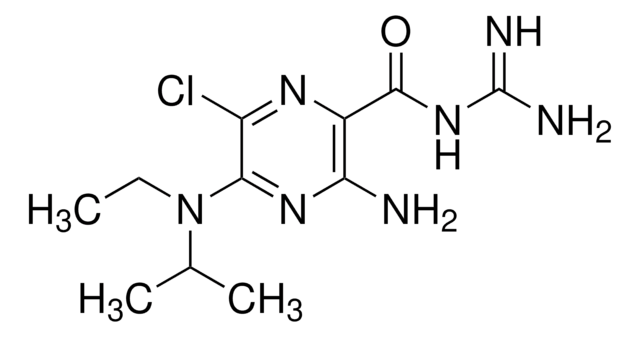
Amiloride hydrochloride hydrate
≥98% (HPLC), powder, T-type calcium channel blocker
Sinônimo(s):
N-Amidino-3,5-diamino-6-chloropyrazinecarboxamide hydrochloride hydrate
About This Item
Produtos recomendados
Nome do produto
Amiloride hydrochloride hydrate, ≥98% (HPLC), powder
Nível de qualidade
Ensaio
≥98% (HPLC)
Formulário
powder
cor
yellow
pf
285-288 °C (dec.)
solubilidade
H2O: 50 mg/mL, clear, yellow-green
originador
Perrigo
temperatura de armazenamento
room temp
cadeia de caracteres SMILES
O.Cl.NC(=N)NC(=O)c1nc(Cl)c(N)nc1N
InChI
1S/C6H8ClN7O.ClH.H2O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11;;/h(H4,8,9,13)(H4,10,11,14,15);1H;1H2
chave InChI
WDZJJRLYFQNCQL-UHFFFAOYSA-N
Informações sobre genes
human ... ABP1(26) , ACCN1(40) , ACCN2(41) , PLAU(5328) , SCNN1A(6337) , SCNN1B(6338) , SCNN1D(6339) , SCNN1G(6340) , SLC9A1(6548) , TNF(7124)
mouse ... Abp1(76507) , Accn1(11418) , Accn2(11419) , Plau(18792) , Scnn1a(20276) , Scnn1b(20277) , Scnn1d(140501) , Scnn1g(20278) , Slc9a1(20544)
rat ... Abp1(65029) , Accn1(25364) , Accn2(79123) , Plau(25619) , Scnn1a(25122) , Scnn1b(24767) , Scnn1g(24768) , Slc9a1(24782)
Aplicação
Ações bioquímicas/fisiológicas
Características e benefícios
Atenção
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Conteúdo relacionado
Discover Bioactive Small Molecules for ADME/Tox
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica