TZHADA210
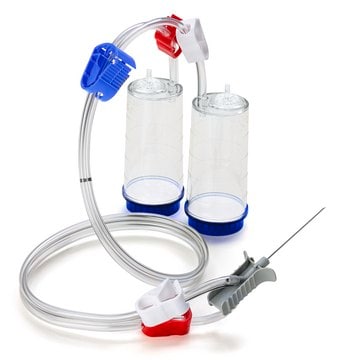
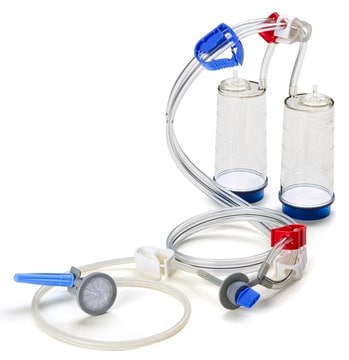
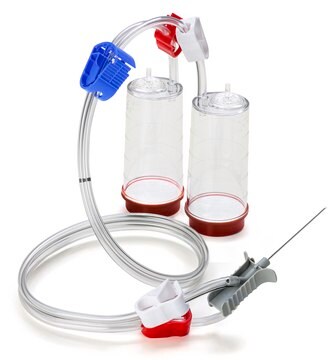
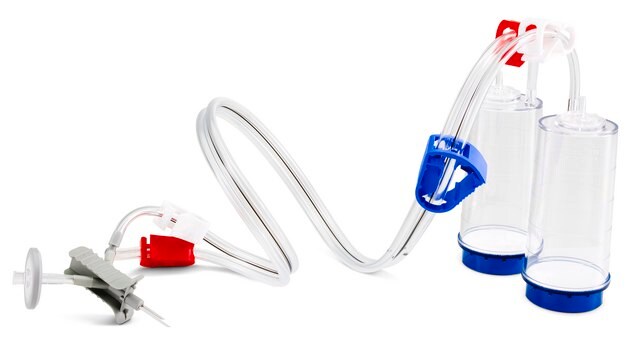
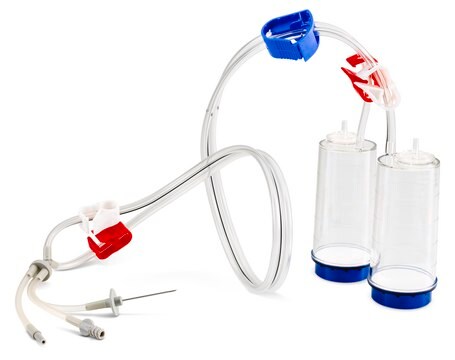
Steritest® NEO Device
For soluble powders in ampoules. Blue base canister with a single needle for transfer into and out of ampoules.
Sinônimo(s):
Blue Base Steritest® NEO device for sterility testing, Sterility testing device, membrane filtration device, membrane filtration canister, closed membrane filtration
About This Item
Produtos recomendados
Materiais
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
mixed cellulose esters (MCE) membrane
plain filter
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
Nível de qualidade
Agency
EP (2.6.1)
JP (4.06)
USP 71
esterilidade
sterile; γ-irradiated
fabricante/nome comercial
Steritest®
embalagem
pkg of 10 blisters per box, Single packed
Parâmetros
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
C tubulação
850 mm
cor
blue Canister Base
matriz
MF-Millipore™
tamanho de poro
0.45 μm pore size
entrada
sample type pharmaceutical(s)
aplicação(ões)
pharmaceutical
sterility testing
Condições de expedição
ambient
Descrição geral
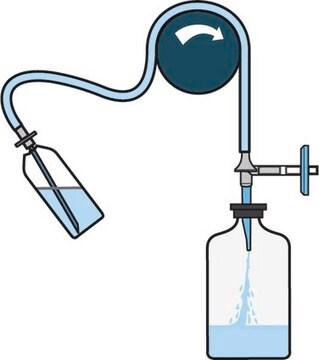
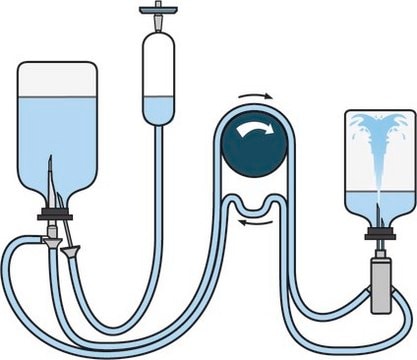
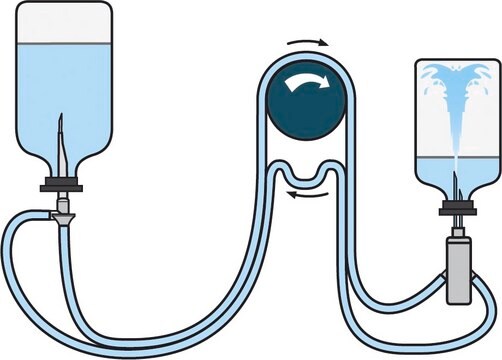
Steritest® NEO Device is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system minimizes false positives, offers the highest levels of quality and reliability, and ensures that pharmaceutical products are never exposed to the environment during the testing process. This test system offers an optimized and fully regulatory compliant testing process, when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The device has vented double needle and simultaneously dissolves/dilutes the sample in sterile diluent and transfers the resulting solution to canisters for further filtration. The blue based canisters indicate mixed cellulose esters (MCE) membrane, which provides an optimal filtration flow rate for standard products.
Aplicação
Características e benefícios
- One-stop-shop for sterility testing with our devices, pumps, media, fluids, and services
- Steritest® devices are manufactured in our Center of Excellence in Molsheim, France, with high-quality control standards maintaining the Certificate of Quality for each lot.
- New needle design
- Smarter workflow
- Completely closed set up
- Consistent performance
- New tubing disconnection tool
Embalagem
Informações legais
configurado para
necessário, mas não fornecido
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica