W241512
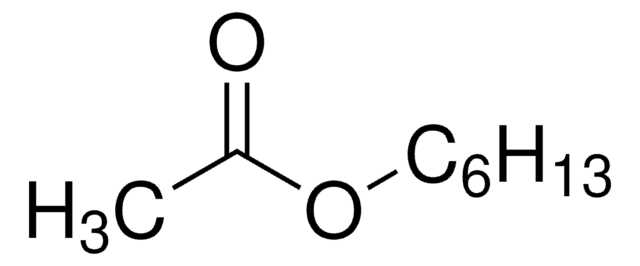
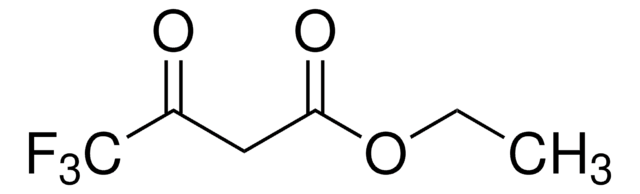
Ethyl acetoacetate
natural, ≥97%, FG
Sinônimo(s):
Acetoacetic ester
About This Item
Produtos recomendados
grau
FG
Fragrance grade
Halal
Kosher
natural
Agency
follows IFRA guidelines
conformidade reg.
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
densidade de vapor
4.48 (vs air)
pressão de vapor
1 mmHg ( 28.5 °C)
Ensaio
≥97%
temperatura de autoignição
580 °F
Lim. expl.
9.5 %
características do produto alternativo mais ecológico
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
índice de refração
n20/D 1.418-1.421
p.e.
181 °C (lit.)
pf
−43 °C (lit.)
solubilidade
water: soluble 35 part
organic solvents: soluble
densidade
1.029 g/mL at 20 °C (lit.)
aplicação(ões)
flavors and fragrances
Documentação
see Safety & Documentation for available documents
alérgeno alimentar
no known allergens
alérgeno de fragrância
no known allergens
categoria alternativa mais ecológica
Organoléptico
apple; fatty; green; fruity
cadeia de caracteres SMILES
CCOC(=O)CC(C)=O
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
chave InChI
XYIBRDXRRQCHLP-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- Benchtop (19)F Nuclear Magnetic Resonance (NMR) Spectroscopy Provides Mechanistic Insight into the Biginelli Condensation toward the Chemical Synthesis of Novel Trifluorinated Dihydro- and Tetrahydropyrimidinones as Antiproliferative Agents.: This study explores the use of Ethyl acetoacetate in the synthesis of novel trifluorinated compounds with potential antiproliferative properties against cancer cells. The research employs advanced NMR spectroscopy to elucidate the reaction mechanism (Chen et al., 2023, Chen et al., 2023).
- Synthesis and Characterization of New Dihydronaphthalene Candidates as Potent Cytotoxic Agents against MCF-7 Human Cancer Cells.: This article discusses the creation of dihydronaphthalene derivatives using Ethyl acetoacetate, highlighting their significant cytotoxic activity against breast cancer cells. The synthesized compounds show promise for further development as chemotherapeutic agents (Ahmed et al., 2020, Ahmed et al., 2020).
- Synthesis and characterization of new 4H-chromene-3-carboxylates ensuring potent elastase inhibition activity along with their molecular docking and chemoinformatics properties.: Utilizing Ethyl acetoacetate, this research focuses on developing 4H-chromene derivatives that exhibit strong elastase inhibition, a key enzyme implicated in various inflammatory diseases. The study integrates molecular docking and chemoinformatics for detailed analysis (Dige et al., 2020, Dige et al., 2020).
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
164.3 °F - closed cup
Ponto de fulgor (°C)
73.5 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W241512-100G-K | 4061834404071 |
| W241512-1KG-K | 4061837876431 |
| W241512-5KG | |
| W241512-100G | |
| W241512-1DRUM-K | |
| W241512-1KG | |
| W241512-5KG-K | 4061837512285 |
| W241512-SAMPLE | |
| W241512-SAMPLE-K | 4061837876448 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica