914436
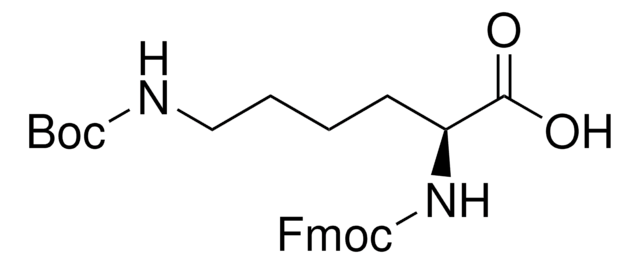
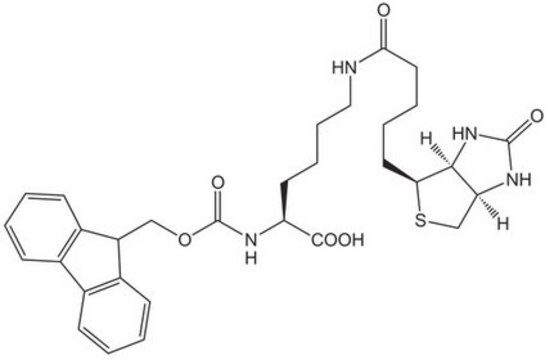
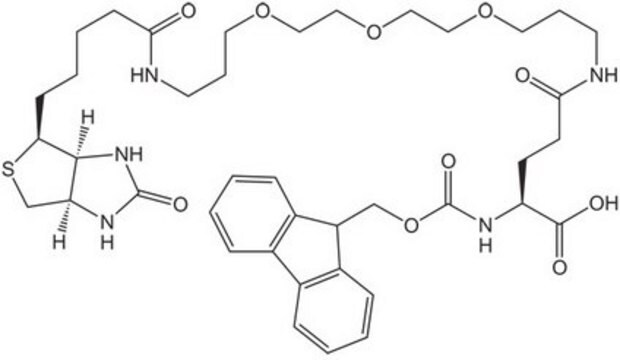
Fmoc-D-Lys(Biotin)-OH
≥95%
Sinônimo(s):
Nα-(9-Fluorenylmethyloxycarbonyl)-Nε-biotinyl-D-lysine, N2-(((9H-Fluoren-9-yl)methoxy)carbonyl)-N6-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoyl)-D-lysine, Biotinylated Fmoc-protected D-lysine
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥95%
Formulário
powder
pf
184-190 °C
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
S1[C@H]([C@H]5NC(=O)N[C@H]5C1)CCCCC(=O)NCCCC[C@@H](NC(=O)OCC2c3c(cccc3)c4c2cccc4)C(=O)O
InChI
1S/C31H38N4O6S/c36-27(15-6-5-14-26-28-25(18-42-26)33-30(39)35-28)32-16-8-7-13-24(29(37)38)34-31(40)41-17-23-21-11-3-1-9-19(21)20-10-2-4-12-22(20)23/h1-4,9-12,23-26,28H,5-8,13-18H2,(H,32,36)(H,34,40)(H,37,38)(H2,33,35,39)/t24-,25+,26+,28+/m1/s1
chave InChI
OFIBQNGDYNGUEZ-KAHNYGAISA-N
Aplicação
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 914436-100MG | 4061842093489 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica