907294
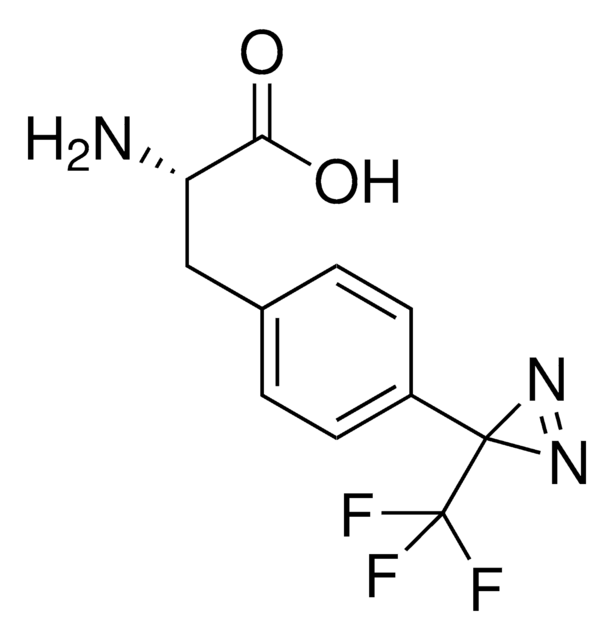
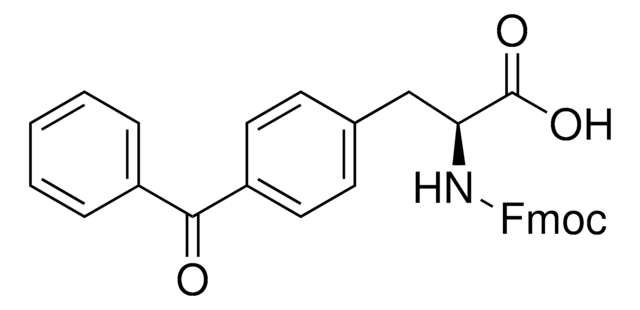
Fmoc-L-Photo-Phe-OH
≥95%
Sinônimo(s):
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid, N-α-(9-Fluorenylmethyloxycarbonyl)-4-(trifluoromethyldiazirin)-L-phenylalanine, Diazirine amino acid, Fmoc-Tdf-OH, Photo-Phe, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Produtos recomendados
Ensaio
≥95%
Formulário
powder
adequação da reação
reaction type: Fmoc solid-phase peptide synthesis
aplicação(ões)
peptide synthesis
grupo funcional
Fmoc
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
FC(F)(F)C5(N=N5)c1ccc(cc1)C[C@H](NC(=O)OCC2c3c(cccc3)c4c2cccc4)C(=O)O
InChI
1S/C26H20F3N3O4/c27-26(28,29)25(31-32-25)16-11-9-15(10-12-16)13-22(23(33)34)30-24(35)36-14-21-19-7-3-1-5-17(19)18-6-2-4-8-20(18)21/h1-12,21-22H,13-14H2,(H,30,35)(H,33,34)/t22-/m0/s1
chave InChI
UHXIAQUVGJCYSA-QFIPXVFZSA-N
Categorias relacionadas
Aplicação
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Outras notas
Mode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass Spectrometry
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
Simple and Versatile Method for Tagging Phenyldiazirine Photophores
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 907294-50MG | 4022536043357 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica