907391
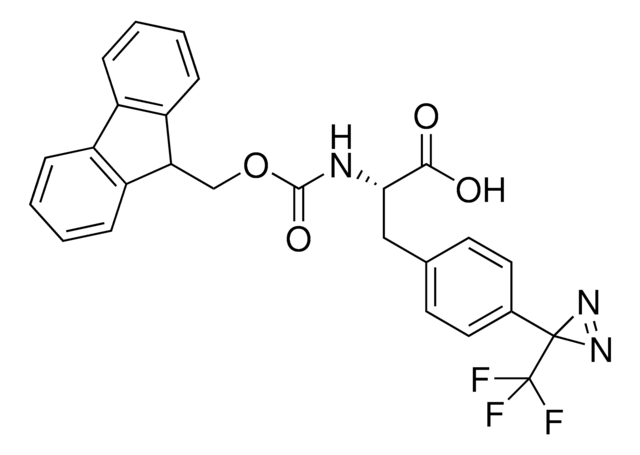
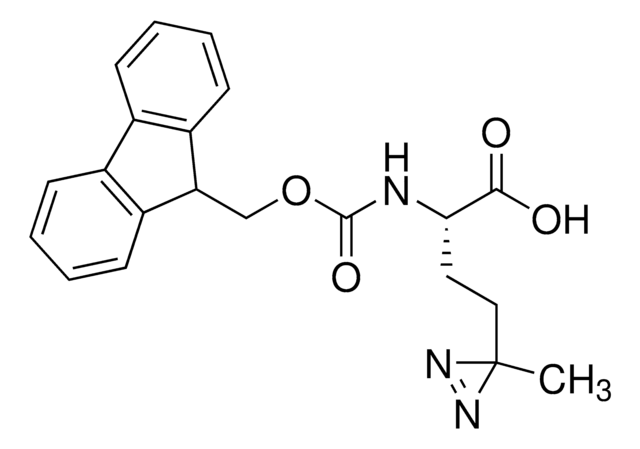
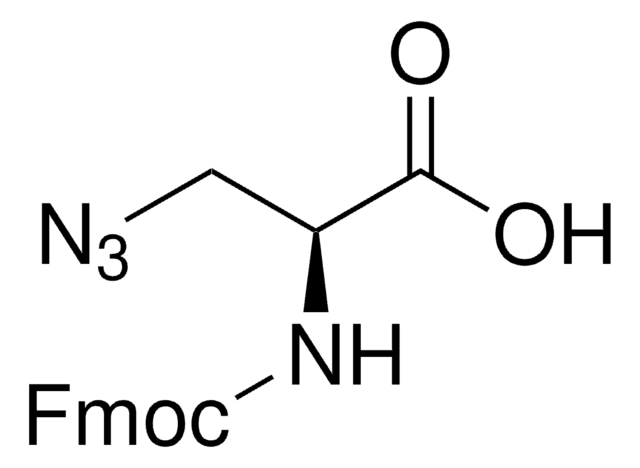
Fmoc-L-photo-leucine
≥98%
Sinônimo(s):
(S)-2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)-3-(3-methyl-3H-diazirin-3-yl)propanoic acid, (S)-2-(Fmoc-amino)-3-(3H-diazirin-3-yl)butanoic acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Produtos recomendados
Ensaio
≥98%
Formulário
powder
adequação da reação
reaction type: Fmoc solid-phase peptide synthesis
aplicação(ões)
peptide synthesis
grupo funcional
Fmoc
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
N([C@@H](CC4(N=N4)C)C(=O)O)C(=O)OCC1c2c(cccc2)c3c1cccc3
InChI
1S/C20H19N3O4/c1-20(22-23-20)10-17(18(24)25)21-19(26)27-11-16-14-8-4-2-6-12(14)13-7-3-5-9-15(13)16/h2-9,16-17H,10-11H2,1H3,(H,21,26)(H,24,25)/t17-/m0/s1
chave InChI
GDWMJFRPAHGSDU-KRWDZBQOSA-N
Aplicação
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Outras notas
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers′
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica