78194
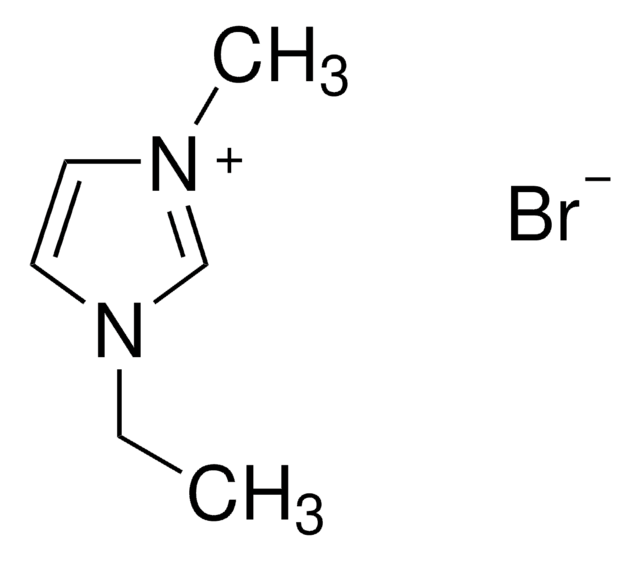
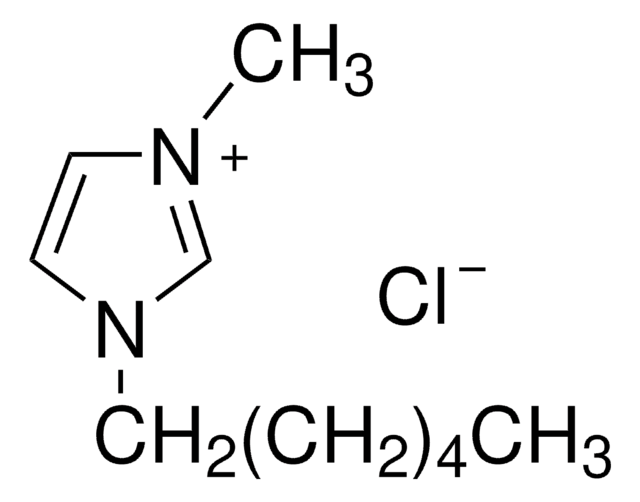
1-Butyl-2,3-dimethylimidazolium chloride
≥97.0% (HPLC/AT)
About This Item
Produtos recomendados
Ensaio
≥97.0% (HPLC/AT)
Formulário
crystals
Impurezas
≤1.0% water
cadeia de caracteres SMILES
[Cl-].CCCCn1cc[n+](C)c1C
InChI
1S/C9H17N2.ClH/c1-4-5-6-11-8-7-10(3)9(11)2;/h7-8H,4-6H2,1-3H3;1H/q+1;/p-1
chave InChI
HHHYPTORQNESCU-UHFFFAOYSA-M
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
- As a solvent in the chemical modification of polysaccharide cellulose.
- As a model ionic liquid in the conversion of a monosaccharide like fructose into 5-hydroxymethylfurfural using H2SO4.
- To prepare 1-butyl-2,3-dimethylimidazolium dicarba-7,8-nidoundecaborate by reacting with caesium dicarba-7,8-nido-undecaborate.
- To prepare mesoporous ZnAl2O4 nanomaterials, which are used as catalysts or catalyst supports.
forma física
Outras notas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Task-Specific Ionic Liquids (TSILs) for the selective liquid/liquid extraction of heavy metals from aqueous systems were first published by Robin D. Rogers et al. in the year 2001.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica