89483
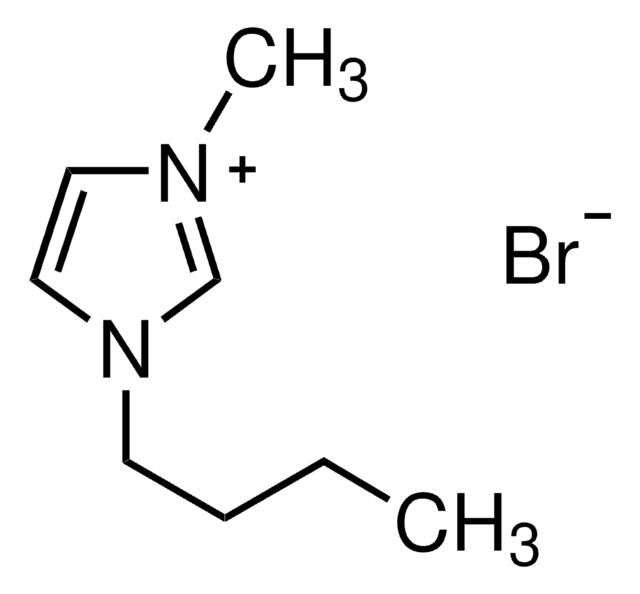
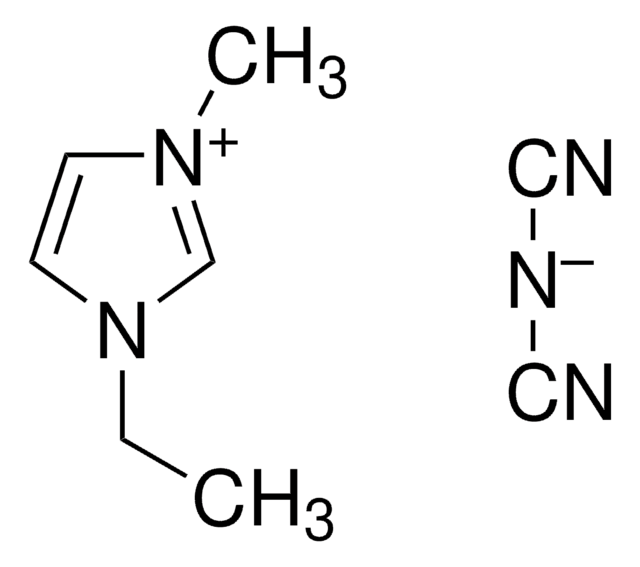
1-Ethyl-3-methylimidazolium bromide
≥97.0% (T)
Sinônimo(s):
1-Ethyl-3-methyl-1H-imidazolium bromide, 1-Methyl-3-ethylimidazolium bromide
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6H11BrN2
Número CAS:
Peso molecular:
191.07
Beilstein:
5162084
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥97.0% (T)
forma
crystals
cadeia de caracteres SMILES
[Br-].CCn1cc[n+](C)c1
InChI
1S/C6H11N2.BrH/c1-3-8-5-4-7(2)6-8;/h4-6H,3H2,1-2H3;1H/q+1;/p-1
chave InChI
GWQYPLXGJIXMMV-UHFFFAOYSA-M
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
1-Ethyl-3-methylimidazolium bromide can be used to prepare:
- 1-ethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide
- 1-ethyl-3-methylimidazolium tetrafluoroborate
- (EMI)[Cd(BTC)] (EMI = 1-ethyl-3-methylimidazolium, BTC = 1,3,5-benzenetricarboxylate), a coordination polymer
Outras notas
Hydrophobic, highly conductive imidazolium molten salt melting at ambient temp.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Voltammetry of oxygen in the room-temperature ionic liquids 1-ethyl-3-methylimidazolium bis ((trifluoromethyl) sulfonyl) imide and hexyltriethylammonium bis ((trifluoromethyl) sulfonyl) imide: one-electron reduction to form superoxide. Steady-state and transient behavior......
Buzzeo MC, et al.
The Journal of Physical Chemistry A, 107(42), 8872-8878 (2003)
The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals.
J. Chem. Soc., Dalton Trans., 13, 2133-2140 (1999)
Ionic liquid as solvent for the synthesis and crystallization of a coordination polymer:(EMI)[Cd(BTC)](EMI=1-ethyl-3-methylimidazolium,BTC=1,3,5 benzenetricarboxylate).
Liao JH, et al.
Crystal Growth & Design, 6(5), 1062-1063 (2006)
Seungmin Oh et al.
The journal of physical chemistry. B, 123(39), 8274-8284 (2019-09-11)
Ionic liquids with aprotic heterocyclic anions (AHAs) have been developed for CO2 capture but have been considered for other applications as well. Previously, we have shown that AHA ILs, where the only site for reaction with CO2 is the anion
Pierre Bonhôte et al.
Inorganic chemistry, 35(5), 1168-1178 (1996-02-28)
New, hydrophobic ionic liquids with low melting points (<-30 degrees C to ambient temperature) have been synthesized and investigated, based on 1,3-dialkyl imidazolium cations and hydrophobic anions. Other imidazolium molten salts with hydrophilic anions and thus water-soluble are also described.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica