463558
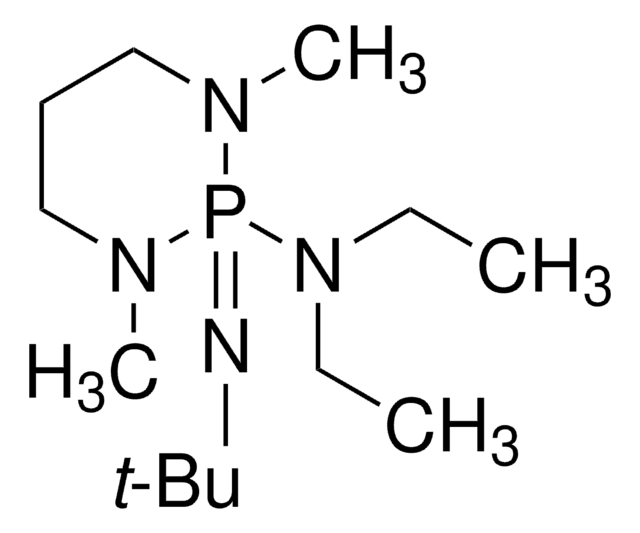
2,8,9-Trimethyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane
Sinônimo(s):
Verkade superbase
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C9H21N4P
Número CAS:
Peso molecular:
216.26
Beilstein:
4970285
Número MDL:
Código UNSPSC:
12352005
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
pf
110-115 °C (lit.)
Nível de qualidade
cadeia de caracteres SMILES
CN1CCN2CCN(C)P1N(C)CC2
InChI
1S/C9H21N4P/c1-10-4-7-13-8-5-11(2)14(10)12(3)6-9-13/h4-9H2,1-3H3
chave InChI
PCYSWBQHCWWSFW-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
2,8,9-Trimethyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane is involved as a reactant in:
- Studies of N-heterocyclic carbene ligand effects on metal hydride bond energies.
- Synthesis of heterogenous basic catalysts immobilized on SBA-15 silica.
- Protection / deprotection strategies for diazeniumdiolate chemistry.
- Encaging of the molecule to study the change in its catalytic ability.
- C-N coupling reactions.
- Derivative synthesis used as a promoter for aza and thia-Michael reaction and Strecker reaction.
Reactant involved in:
- Studies of N-heterocyclic carbene ligand effects on metal hydride bond energies
- Synthesis of heterogenous basic catalysts immobilized on SBA-15 silica
- Protection / deprotection strategies for diazeniumdiolate chemistry
- Encaging of the molecule to study change in its catalytic ability
- C-N coupling reactions
- Derivative synthesis used as a promoter for aza and thia-Michael reaction and Strecker reaction
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
A new step towards solid base catalysis: azidoproazaphosphatranes immobilized in nanopores of mesoporous silica.
Raytchev P D, et al.
Advanced Synthesis & Catalysis, 353(11?12), 2067-2077 (2011)
Novel protection?deprotection strategies in diazeniumdiolate chemistry: synthesis of V-IPA/NO.
Nandurdikar R S, et al.
Chemical Communications (Cambridge, England), 47(23), 6710-6712 (2011)
Comprehensive Thermochemistry of W?H Bonding in the Metal Hydrides CpW (CO) 2 (IMes) H,[CpW (CO) 2 (IMes) H]?+, and [CpW (CO) 2 (IMes)(H) 2]+. Influence of an N-Heterocyclic Carbene Ligand on Metal Hydride Bond Energies.
Roberts J A, et al.
Journal of the American Chemical Society, 133(37), 14604-14613 (2011)
(t-Bu) 2PN P (i-BuNCH2CH2) 3N: New Efficient Ligand for Palladium-Catalyzed C? N Couplings of Aryl and Heteroaryl Bromides and Chlorides and for Vinyl Bromides at Room Temperature.
Reddy C V, et al.
The Journal of Organic Chemistry, 73(8), 3047-3062 (2008)
Encaging the Verkade?s superbases: thermodynamic and kinetic consequences.
Raytchev P D, et al.
Journal of the American Chemical Society, 133(7), 2157-2159 (2011)
Artigos
An article on Proazaphosphatranes: Verkade’s Superbases.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica![2,8,9-Triisopropyl-2,5,8,9-tetraaza-1-phosphabicyclo[3,3,3]undecane](/deepweb/assets/sigmaaldrich/product/structures/387/021/edaffe12-6e4b-4305-9030-749551ac828a/640/edaffe12-6e4b-4305-9030-749551ac828a.png)
![2,8,9-Triisobutyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane 97%](/deepweb/assets/sigmaaldrich/product/structures/750/287/cc77a98e-fa6c-4d81-9f3e-f392770724ac/640/cc77a98e-fa6c-4d81-9f3e-f392770724ac.png)
![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)