Key Documents
A3656
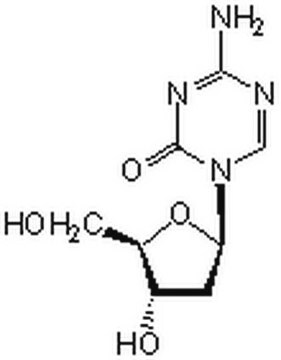
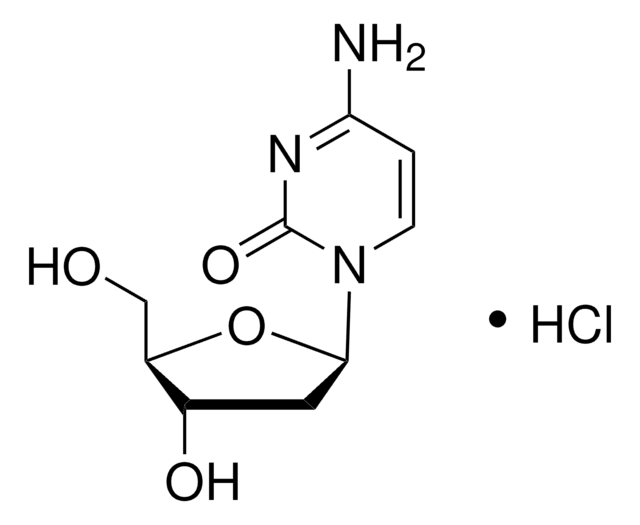
5-Aza-2′-deoxycytidine
≥97% (HPLC), powder, DNA methyltransferase inhibitor
Synonim(y):
2′-Deoxy-5-azacytidine, 4-Amino-1-(2-deoxy-β-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one, Decitabine
About This Item
Polecane produkty
product name
5-Aza-2′-deoxycytidine, ≥97%
Próba
≥97%
Postać
powder
rozpuszczalność
acetic acid: water (1:1): 50 mg/mL
inicjator
Eisai
ciąg SMILES
NC1=NC(=O)N(C=N1)[C@H]2C[C@H](O)[C@@H](CO)O2
InChI
1S/C8H12N4O4/c9-7-10-3-12(8(15)11-7)6-1-4(14)5(2-13)16-6/h3-6,13-14H,1-2H2,(H2,9,11,15)/t4-,5+,6+/m0/s1
Klucz InChI
XAUDJQYHKZQPEU-KVQBGUIXSA-N
informacje o genach
human ... DNMT1(1786) , DNMT3A(1788)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Cechy i korzyści
Uwaga dotycząca przygotowania
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Eye Irrit. 2 - Muta. 2 - Repr. 1B - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Bis-Tris gels and buffers for superior protein resolution compared to traditional tris-glycine gels.
Żele i bufory Bis-Tris zapewniają lepszą rozdzielczość białek w porównaniu z tradycyjnymi żelami tris-glicynowymi.
Carcinogenesis and Epigenetics
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej
![Adenosine 5′-[γ-thio]triphosphate tetralithium salt ≥75% (HPLC), powder](/deepweb/assets/sigmaaldrich/product/structures/319/398/e29221c2-3649-455b-bd33-583bb017ec7d/640/e29221c2-3649-455b-bd33-583bb017ec7d.png)