Wszystkie zdjęcia(6)
Kluczowe dokumenty
D3897
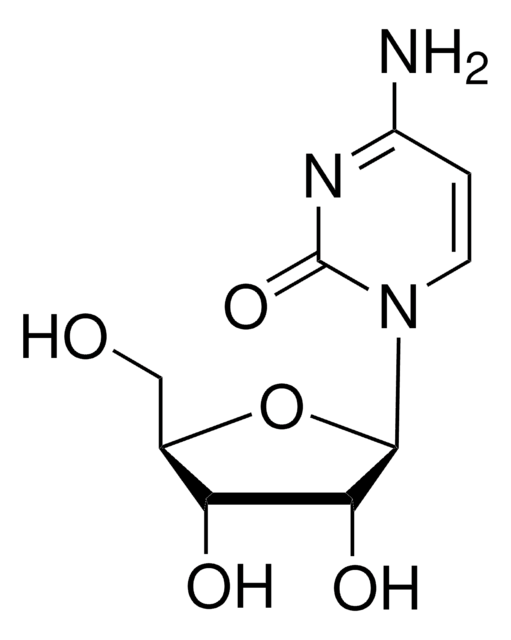
2′-Deoxycytidine
≥99% (HPLC)
Synonim(y):
Cytosine deoxyriboside
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C9H13N3O4
Numer CAS:
Masa cząsteczkowa:
227.22
Beilstein:
87567
Numer WE:
Numer MDL:
Kod UNSPSC:
41106305
Identyfikator substancji w PubChem:
NACRES:
NA.51
Polecane produkty
pochodzenie biologiczne
synthetic (organic)
Próba
≥99% (HPLC)
Formularz
powder
rozpuszczalność
water: 50 mg/mL, clear, colorless to very faintly yellow
temp. przechowywania
−20°C
ciąg SMILES
NC1=NC(=O)N(C=C1)[C@H]2C[C@H](O)[C@@H](CO)O2
InChI
1S/C9H13N3O4/c10-7-1-2-12(9(15)11-7)8-3-5(14)6(4-13)16-8/h1-2,5-6,8,13-14H,3-4H2,(H2,10,11,15)/t5-,6+,8+/m0/s1
Klucz InChI
CKTSBUTUHBMZGZ-SHYZEUOFSA-N
Opis ogólny
2′-Deoxycytidine (deoxyC) is one of the deoxynucleosides containing cytosine as the nucleobase. It is found in the blood, feces, and urine.
Zastosowanie
2′-Deoxycytidine has been used:
- as a substrate for Trypanosoma brucei cytidine deaminase (TbCDA) to measure its activity
- as a standard in the isolation and quantification of metabolite levels in murine tumor interstitial fluid by liquid chromatography-mass spectrometry (LC–MS)
- to study the role of autophagy in response to oncogenes and DNA replication stress
Działania biochem./fizjol.
2′-Deoxycytidine (deoxyC) forms dCTP upon phosphorylation which is used to synthesis DNA via various DNA polymerases or reverse transcriptases. DeoxyC is the substrate for deoxycytidine deaminase (EC 3.5.4.14) which converts it into 2′-deoxyuridine. DeoxyC is phosphorylated to the nucleotide dCMP by the enzyme deoxycytidine kinase (DCK). DeoxyC serves as a potential head and neck cancer marker.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Hiroya Murakami et al.
Talanta, 177, 12-17 (2017-11-08)
Acetaldehyde (AA), which is present in tobacco smoke, automobile exhaust gases and alcohol beverage, is a mutagen and carcinogen. AA reacts with 2'-deoxyguanosine (dG) in DNA to form N
Martyna Modrzejewska et al.
Free radical biology & medicine, 101, 378-383 (2016-11-12)
The most plausible mechanism behind active demethylation of 5-methylcytosine involves TET proteins which participate in oxidation of 5-methylcytosine to 5-hydroxymethylcytosine; the latter is further oxidized to 5-formylcytosine and 5-carboxycytosine. 5-Hydroxymethyluracil can be also generated from thymine in a TET-catalyzed process.
Annika R Seddon et al.
Epigenetics & chromatin, 14(1), 17-17 (2021-03-26)
Environmental factors, such as oxidative stress, have the potential to modify the epigenetic landscape of cells. We have previously shown that DNA methyltransferase (DNMT) activity can be inhibited by sublethal doses of hydrogen peroxide (H2O2). However, site-specific changes in DNA
Santiago Uribe-Lewis et al.
Genome biology, 16, 69-69 (2015-04-09)
The discovery of cytosine hydroxymethylation (5hmC) as a mechanism that potentially controls DNA methylation changes typical of neoplasia prompted us to investigate its behaviour in colon cancer. 5hmC is globally reduced in proliferating cells such as colon tumours and the
E Mini et al.
Annals of oncology : official journal of the European Society for Medical Oncology, 17 Suppl 5, v7-12 (2006-06-30)
Gemcitabine (2',2'-difluoro 2'-deoxycytidine, dFdC) is the most important cytidine analogue developed since cytosine arabinoside (Ara-C). The evidence of its potent antitumor activity in a wide spectrum of in vitro and in vivo tumor models has been successfully confirmed in the
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej