Key Documents
T1952
Trichostatin A, Ready Made Solution
5 mM in DMSO, from Streptomyces sp.
Synonim(y):
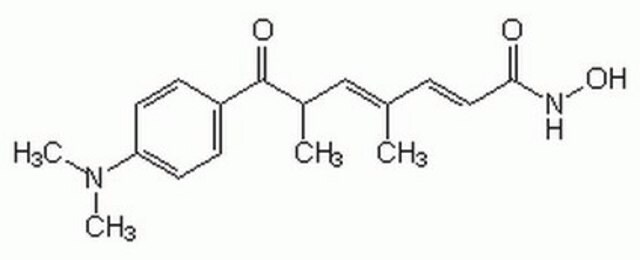
TSA, [R-(E,E)]-7-[4-(Dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide
About This Item
Polecane produkty
pochodzenie biologiczne
Streptomyces sp.
Poziom jakości
Próba
≥98% (HPLC)
Postać
DMSO solution
stężenie
5 mM in DMSO
metody
cell culture | mammalian: suitable
spektrum działania antybiotyku
fungi
neoplastics
Tryb działania
enzyme | inhibits
Warunki transportu
dry ice
temp. przechowywania
−20°C
InChI
1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+
Klucz InChI
RTKIYFITIVXBLE-WKWSCTOISA-N
Opis ogólny
Zastosowanie
- as a histone deacetylase inhibitor to study its effect on transcriptome changes by stem cell testing
- to inhibit histone deacetylase (HDAC) class I, II, or III in primary pituitary cell cultures and to investigate insulin control of endogenous human growth hormone gene (hGH)
- to treat cells for the HDAC inhibition experiments
Działania biochem./fizjol.
produkt powiązany
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej