Kluczowe dokumenty
700015P
Avanti
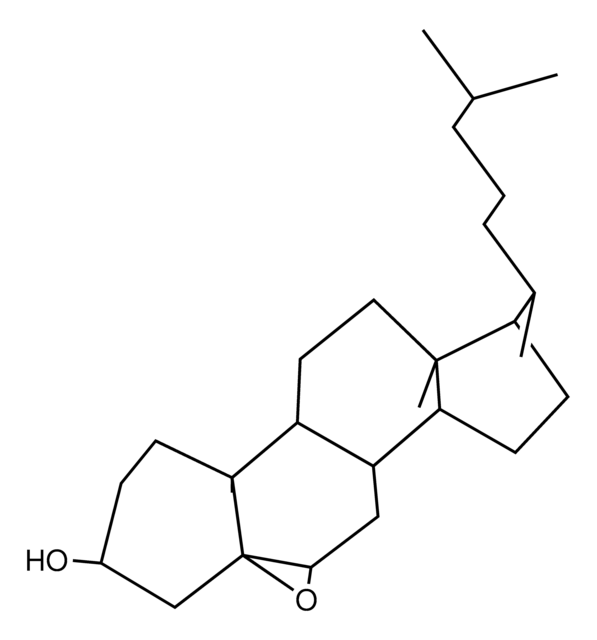
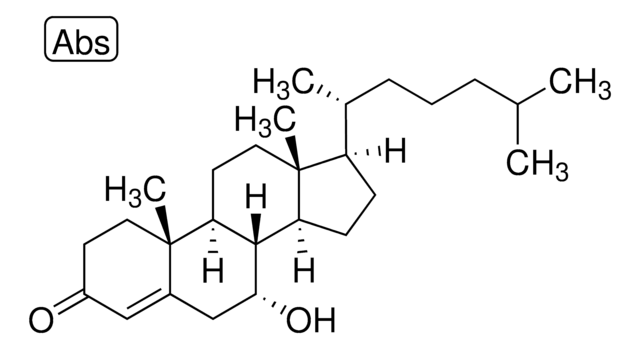
7-ketocholesterol
Avanti Research™ - A Croda Brand
Synonim(y):
7-oxocholesterol; 7-oxo-5-cholesten-3β-ol-7-one; 5-cholesten-3β-ol-7-one; 3β-hydroxycholest-5-en-7-one; 110806
About This Item
Polecane produkty
opis
3β-hydroxy-5-cholestene-7-one
Próba
>99% (TLC)
Formularz
powder
opakowanie
pkg of 1 × 100 mg (700015P-100mg)
pkg of 1 × 5 mg (700015P-5mg)
pkg of 1 × 50 mg (700015P-50mg)
producent / nazwa handlowa
Avanti Research™ - A Croda Brand
Warunki transportu
dry ice
temp. przechowywania
−20°C
ciąg SMILES
O[C@H]1CC[C@@]2([C@@H]3[C@H]([C@H]4[C@@]([C@H](CC4)[C@@H](CCCC(C)C)C)(CC3)C)C(=O)C=C2C1)C
InChI
1S/C27H44O2/c1-17(2)7-6-8-18(3)21-9-10-22-25-23(12-14-27(21,22)5)26(4)13-11-20(28)15-19(26)16-24(25)29/h16-18,20-23,25,28H,6-15H2,1-5H3/t18-,20+,21-,22+,23+,25+,26+,27-/m1/s1
Klucz InChI
YIKKMWSQVKJCOP-ABXCMAEBSA-N
Opis ogólny
Zastosowanie
<li><strong>Cytoprotekcyjne działanie kwasu linolenowego na uszkodzenia wywołane 7-ketocholesterolem:</strong> Badanie to podkreśla, w jaki sposób określone kwasy tłuszczowe mogą łagodzić toksyczne działanie 7-ketocholesterolu w komórkach nerwowych i naczyniowych, podkreślając potencjalne zastosowania terapeutyczne w chorobach neurodegeneracyjnych i sercowo-naczyniowych (Yammine et al., 2024).</li>
</ul>.
Działania biochem./fizjol.
Opakowanie
Informacje prawne
najczęściej kupowane z tym produktem
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej