700113P
Avanti
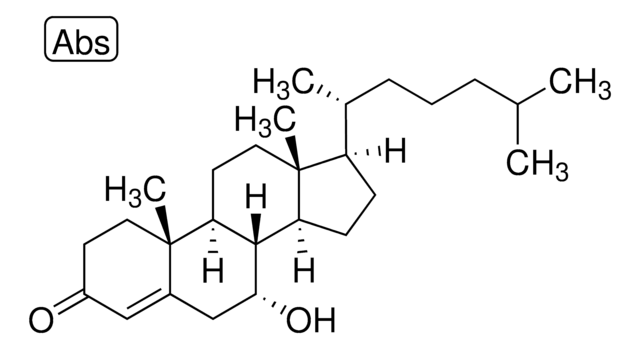
7α-hydroxycholestenone
Avanti Research™ - A Croda Brand
Synonim(y):
C4; α-HC; α-HC; 7HCO; 7 α-3ox-C; 111107
About This Item
Polecane produkty
opis
7α-hydroxy-4-cholesten-3-one
Próba
>99% (TLC)
Formularz
powder
opakowanie
pkg of 1 × 1 mg (700113P-1mg)
pkg of 1 × 10 mg (700113P-10mg)
producent / nazwa handlowa
Avanti Research™ - A Croda Brand
Warunki transportu
dry ice
temp. przechowywania
−20°C
InChI
1S/C27H44O2/c1-17(2)7-6-8-18(3)21-9-10-22-25-23(12-14-27(21,22)5)26(4)13-11-20(28)15-19(26)16-24(25)29/h15,17-18,21-25,29H,6-14,16H2,1-5H3/t18-,21-,22+,23+,24-,25+,26+,27-/m1/s1
Klucz InChI
IOIZWEJGGCZDOL-RQDYSCIWSA-N
Opis ogólny
Zastosowanie
- Role as a mediator in cholesterol metabolism: 7α-hydroxycholestenone has been implicated in the discussion on oxysterols as cholesterol metabolites that act as significant mediators within biological systems, highlighting its importance in cholesterol metabolism and potential pharmaceutical implications (Mutemberezi et al., 2016).
Opakowanie
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej