Kluczowe dokumenty
52853
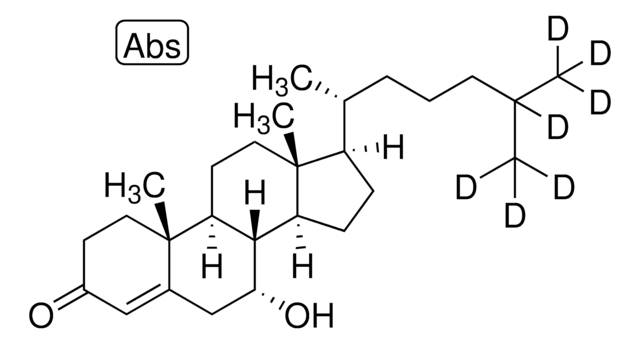
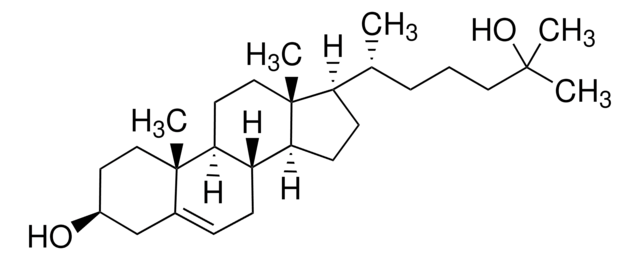
7α-Hydroxy-4-cholesten-3-one
≥95.0% (HPLC)
Synonim(y):
7α-Hydroxycholest-4-en-3-one
About This Item
Polecane produkty
klasa czystości
analytical standard
Poziom jakości
Próba
≥95.0% (HPLC)
Zastosowanie
clinical testing
Format
neat
temp. przechowywania
−20°C
ciąg SMILES
O=C1CC[C@@]2(C)C(C[C@@H](O)[C@]3([H])[C@]2([H])CC[C@@]4(C)[C@@]3([H])CC[C@]4([H])[C@H](C)CCCC(C)C)=C1
InChI
1S/C27H44O2/c1-17(2)7-6-8-18(3)21-9-10-22-25-23(12-14-27(21,22)5)26(4)13-11-20(28)15-19(26)16-24(25)29/h15,17-18,21-25,29H,6-14,16H2,1-5H3/t18-,21-,22+,23+,24-,25+,26+,27-/m1/s1
Klucz InChI
IOIZWEJGGCZDOL-RQDYSCIWSA-N
Opis ogólny
Zastosowanie
- Human serum by ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) with electrospray ionization source (ESI) as well as LC-MS.
- Rat and monkey plasma by LC-ESI-MS/MS working on multiple reaction monitoring (MRM) mode of detection.
- Peripheral blood plasma by solid-phase extraction (SPE) and HPLC.
Działania biochem./fizjol.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej