Kluczowe dokumenty
About This Item
Polecane produkty
Poziom jakości
Próba
98%
Formularz
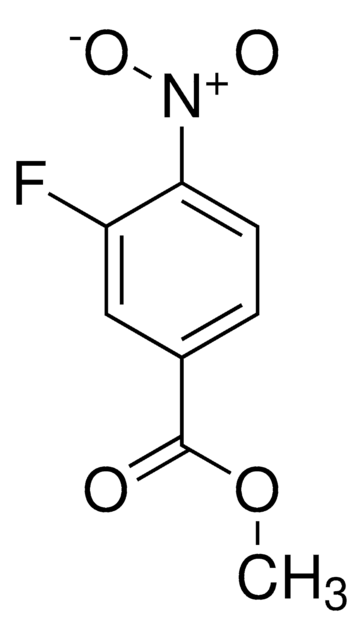
solid
mp
123-126 °C (lit.)
rozpuszczalność
95% ethanol: soluble 50 mg/mL, clear, light yellow
grupa funkcyjna
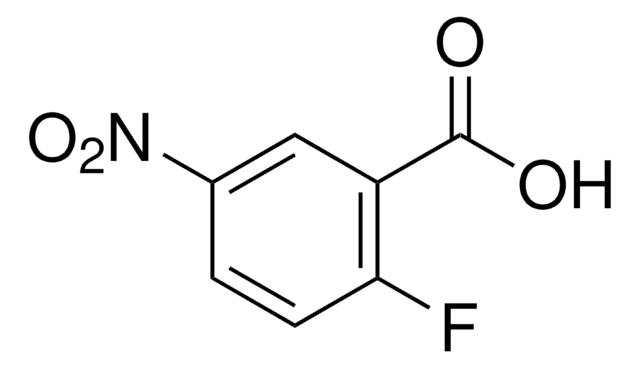
carboxylic acid
fluoro
nitro
ciąg SMILES
OC(=O)c1ccc(F)c(c1)[N+]([O-])=O
InChI
1S/C7H4FNO4/c8-5-2-1-4(7(10)11)3-6(5)9(12)13/h1-3H,(H,10,11)
Klucz InChI
BOJWTAQWPVBIPG-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- as starting reagent in the preparation of novel benzimidazoles having antimycobacterial activity
- in preparation of series of novel acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors containing benzimidazole core structure
- in preparation of bis(heterocyclic) skeletal precursors for the Pictet-Spengler reaction
- in solid-phase synthesis of trisubstituted [1,3,5]triazino[1,2-a]benzimidazole-2,4(3H,10H)-diones
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej