914088
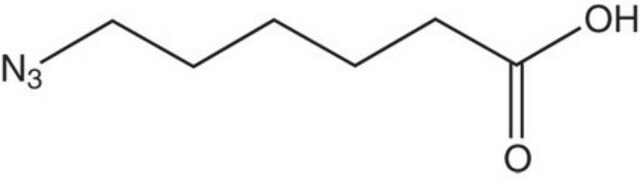
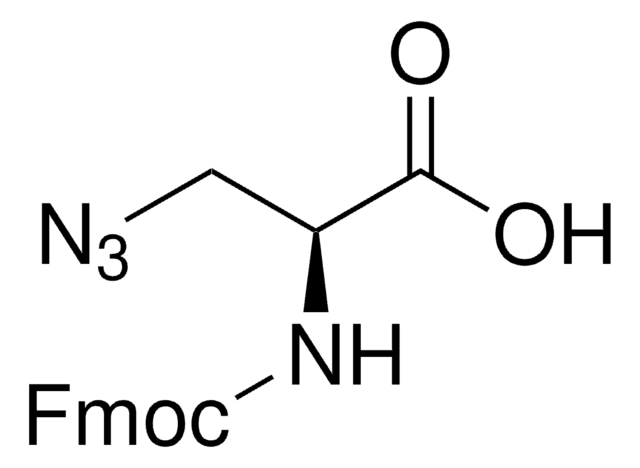
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride
≥95%
別名:
(S)-2-amino-6-((2-azidoethoxy)carbonylamino)hexanoic acid hydrochloride, Clickable amino acid for bioconjugation, H-L-Lys(EO-N3)-OH HCl, Lysine-azide, UAA crosslinker
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
C9H17N5O4 · xHCl
CAS番号:
分子量:
259.26 (free base basis)
MDL番号:
UNSPSCコード:
12352209
おすすめの製品
品質水準
アッセイ
≥95%
フォーム
powder
保管温度
−20°C
SMILES記法
[N+](=[N-])=NCCOC(=O)NCCCC[C@H](N)C(=O)O.C
InChI
1S/C9H17N5O4.CH4/c10-7(8(15)16)3-1-2-4-12-9(17)18-6-5-13-14-11;/h7H,1-6,10H2,(H,12,17)(H,15,16);1H4/t7-;/m0./s1
InChI Key
LQERWAMRZNEGIE-FJXQXJEOSA-N
アプリケーション
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride is a clickable amino acid derivative for site-specific incorporation into recombinant proteins or synthesis of chemical probes and tools for biological applications. This non-canonical lysine possesses an azide for bioorthogonal reaction with alkynes.
その他情報
Construction of bacterial cells with an active transport system for unnatural amino acids
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
関連製品
製品番号
詳細
価格
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Self-react. C
保管分類コード
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
914088-VAR:
914088-250MG:
914088-BULK:
最新バージョンのいずれかを選択してください:
Siqi Zheng et al.
Angewandte Chemie (International ed. in English), 53(25), 6449-6453 (2014-05-16)
Coupling the genetic code expansion technique with bioorthogonal reactions enables precise control over the conjugation site as well as the choice of fluorescent probes during protein labeling. However, the advantages of this strategy over bulky and rigid fluorescent proteins (FPs)
Chuanling Zhang et al.
Biomaterials, 80, 134-145 (2015-12-29)
Virus-based nanoparticles have shown promise as vehicles for delivering therapeutic genes. However, the rational design of viral vectors that enable selective tropism towards particular types of cells and tissues remains challenging. Here, we explored structural-functional relationships of the adeno-associated virus
Sanggil Kim et al.
Bioorganic & medicinal chemistry, 24(22), 5816-5822 (2016-10-26)
Proteins often function as complex structures in conjunction with other proteins. Because these complex structures are essential for sophisticated functions, developing protein-protein conjugates has gained research interest. In this study, site-specific protein-protein conjugation was performed by genetically incorporating an azide-containing
Michael P VanBrunt et al.
Bioconjugate chemistry, 26(11), 2249-2260 (2015-09-04)
Antibody-drug conjugates (ADC) have emerged as potent antitumor drugs that provide increased efficacy, specificity, and tolerability over chemotherapy for the treatment of cancer. ADCs generated by targeting cysteines and lysines on the antibody have shown efficacy, but these products are
Yiming Wu et al.
Bioconjugate chemistry, 27(10), 2460-2468 (2016-10-21)
Radioimmunotherapy (RIT) delivers radioisotopes to antigen-expressing cells via monoantibodies for the imaging of lesions or medical therapy. The chelates are typically conjugated to the antibody through cysteine or lysine residues, resulting in heterogeneous chelate-to-antibody ratios and various conjugation sites. To
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)