おすすめの製品
詳細
Ethylene (ET), a simple olefin, is an important synthetic reagent. It is also a plant hormone that regulates various physiological and developmental processes, such as ripening of fruit, abscission, senescence and responses to wounding in plants. Pd(II) and Ni(II)-based catalysts are reported to catalyze the polymerization of ET to afford high molecular weight polymers. Mechanism of Diels-Alder Reaction of 1,3-butadiene with ET has been investigated. Diels-Alder reaction between butadiene and ET, via concerted and the stepwise mechanisms has been investigated by ab initio MO methods. Dehydration of algae-produced ethanol to ET in the presence of nanoscale catalyst HZSM-5 (protonated Zeolite Socony Mobil-5) shows promising results in replacing fossil fuel methods for the industrial production of ET. The utility of microsized spent tea leaf powder (MSTLP) as an ethylene adsorbent has been investigated.
アプリケーション
Ethylene was used in the preparation of polyethylene (PE), by polymerization in the presence of titanium complexes having F(s) or CF3(s) containing phenoxy-imine chelate ligands.
包装
Supplied in a carbon steel lecture bottle with a CGA180M/CGA110F needle valve installed.
Compatible with the following:
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
その他情報
See Technical Information Bulletin AL-151 Gas Regulators: Selection, Installation, and Operation
法的情報
Aldrich is a registered trademark of Sigma-Aldrich Co. LLC
よく一緒に購入される製品
製品番号
詳細
価格
コントロールバルブ
パージ値
ホースバーブ
レギュレーター
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Flam. Gas 1 - Press. Gas Liquefied gas - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
2A - Gases
WGK
nwg
引火点(°F)
-148.0 °F - closed cup
引火点(℃)
-100 °C - closed cup
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
536164-BULK:
536164-VAR:
536164-110G-PW:
536164-110G:
Ethylene formation by catalytic dehydration of ethanol with industrial considerations.
Fan D, et al.
Materials, 6(1), 101-115 (2012)
Hiroaki Wakayama et al.
The journal of physical chemistry. A, 111(51), 13575-13582 (2007-12-07)
The concerted and the stepwise mechanisms of the Diels-Alder reactions of butadiene with silaethylene and disilene were studied by ab initio MO methods. For the reaction of butadiene and silaethylene, an asymmetric concerted process that is almost stepwise and two
Ethylene and the regulation of plant development.
Schaller GE.
BMC Biology, 10(1), 9-9 (2012)
Spent tea leaves: A new non-conventional and low-cost biosorbent for ethylene removal.
Tzeng JH, et al.
International Biodeterioration & Biodegradation, 104, 67-73 (2015)
Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants.
Hamilton AJ, et al.
Nature, 284-287 (1990)
資料
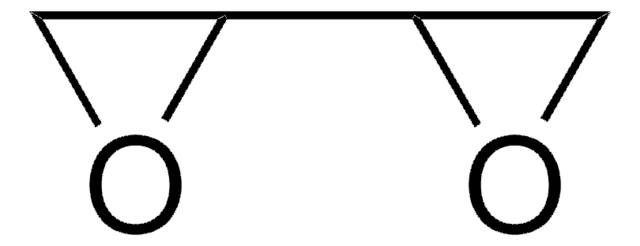
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)