857661
4-Aminobenzamidine dihydrochloride
98%
Sinónimos:
p-Aminobenzimidamide dihydrochloride
Seleccione un Tamaño
Seleccione un Tamaño
About This Item
Productos recomendados
Nivel de calidad
Ensayo
98%
Formulario
crystals
mp
>300 °C (lit.)
grupo funcional
amine
temp. de almacenamiento
2-8°C
cadena SMILES
Cl[H].Cl[H].NC(=N)c1ccc(N)cc1
InChI
1S/C7H9N3.2ClH/c8-6-3-1-5(2-4-6)7(9)10;;/h1-4H,8H2,(H3,9,10);2*1H
Clave InChI
GHEHNICLPWTXJC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
1 of 4
Este artículo | EP0030010060 | EP0030075285 | EP0030010027 |
|---|---|---|---|
| sterility non-sterile | sterility non-sterile | sterility sterile | sterility sterile; γ-irradiated |
| volume (2-200 μL) | volume (50-1000 μL) | volume - | volume (0.2-20 μL) |
| feature individually wrapped | feature individually wrapped | feature DNA free, PCR clean, barrier: no, graduations: no, pyrogen free | feature DNase free, individually wrapped |
| manufacturer/tradename Eppendorf® 0030010043 | manufacturer/tradename Eppendorf® 0030010060 | manufacturer/tradename Eppendorf® 0030075285 | manufacturer/tradename Eppendorf® 0030010027 |
| material colorless | material colorless | material - | material colorless |
| packaging pack of 100 ea (individually wrapped) | packaging pack of 100 ea (individually wrapped), pkg of 100 tips | packaging case of 240 ea (5 racks x 48 tips) | packaging pack of 100 ea (individually wrapped), pkg of 100 tips |
Aplicación
- Orally active fibrinogen receptor antagonists based on benzamidines.[1]
- Benzamidine derivatives that are selective and potent serine protease inhibitors.[2][3]
- Novel pyrrolo [3,2-c] quinolines that are structural analogs of topoisomerase inhibitors such as coralyne and fagaronine.[4]
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
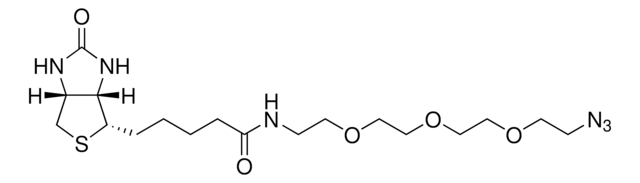


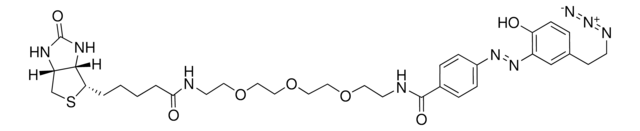
![Tris[(1-benzyl-1H-1, 2, 3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)
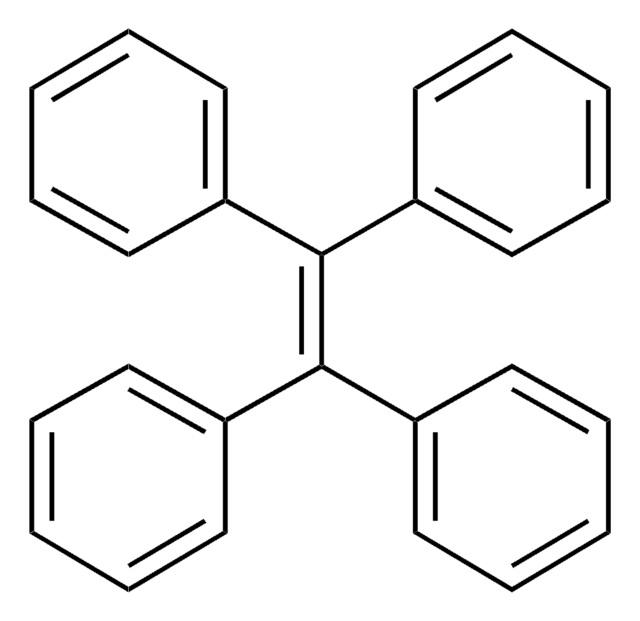
![B-[4-(1,2,2-Triphenylethenyl)phenyl]boronic acid](/deepweb/assets/sigmaaldrich/product/structures/121/044/864e0829-e1de-4170-aae4-16c2b3ce4111/640/864e0829-e1de-4170-aae4-16c2b3ce4111.png)