Wszystkie zdjęcia(2)
Kluczowe dokumenty
857661
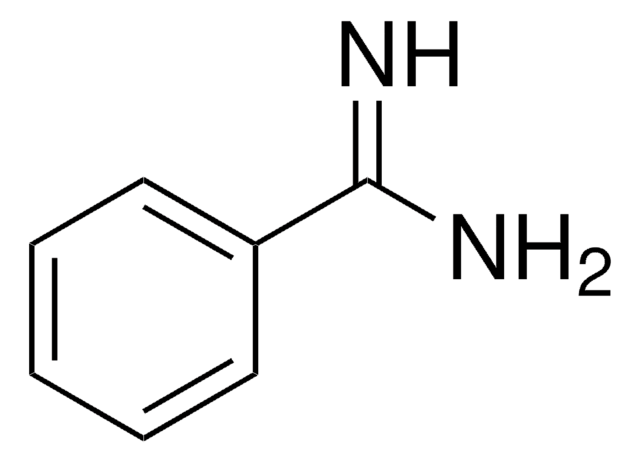
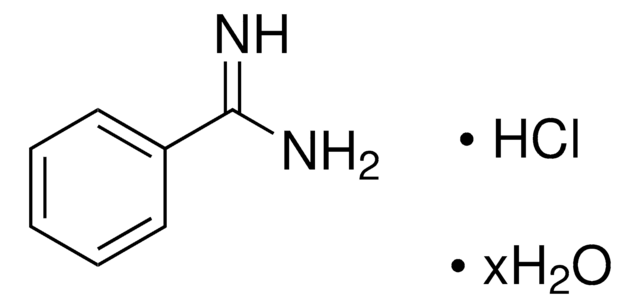
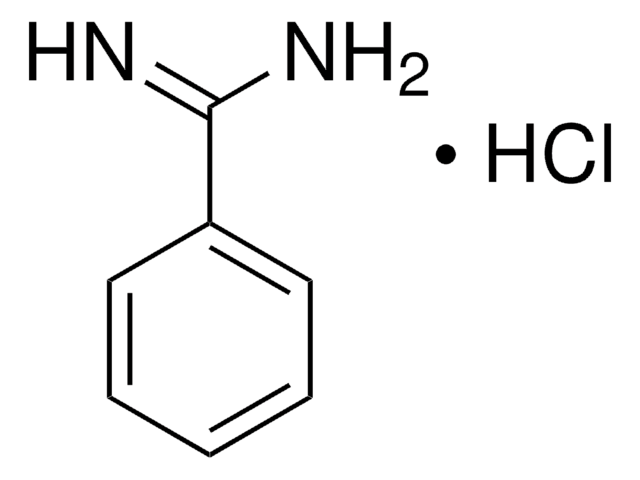
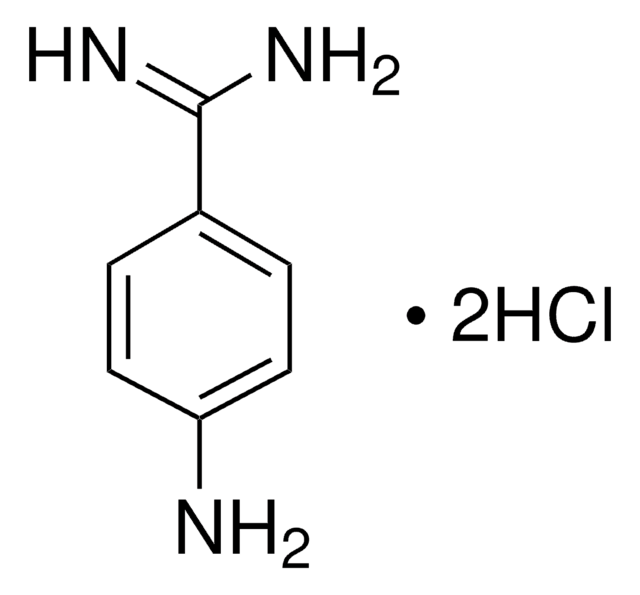
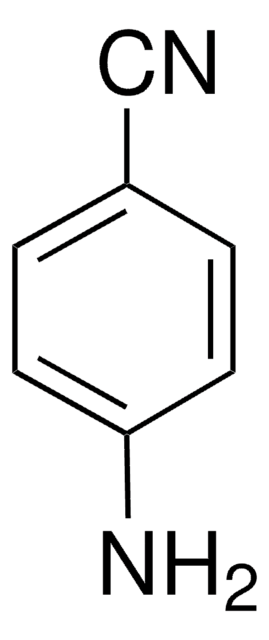
4-Aminobenzamidine dihydrochloride
98%
Synonim(y):
p-Aminobenzimidamide dihydrochloride
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór liniowy:
H2NC6H4C(=NH)NH2·2HCl
Numer CAS:
Masa cząsteczkowa:
208.09
Beilstein:
3692927
Numer WE:
Numer MDL:
Kod UNSPSC:
12352100
Identyfikator substancji w PubChem:
NACRES:
NA.22
Polecane produkty
Próba
98%
Formularz
crystals
mp
>300 °C (lit.)
grupa funkcyjna
amine
temp. przechowywania
2-8°C
ciąg SMILES
Cl[H].Cl[H].NC(=N)c1ccc(N)cc1
InChI
1S/C7H9N3.2ClH/c8-6-3-1-5(2-4-6)7(9)10;;/h1-4H,8H2,(H3,9,10);2*1H
Klucz InChI
GHEHNICLPWTXJC-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
4-Aminobenzamidine dihydrochloride can be used to synthesize:
- Orally active fibrinogen receptor antagonists based on benzamidines.
- Benzamidine derivatives that are selective and potent serine protease inhibitors.
- Novel pyrrolo [3,2-c] quinolines that are structural analogs of topoisomerase inhibitors such as coralyne and fagaronine.
4-Aminobenzamidine dihydrochloride is used as a ligand in affinity chromatography for purification and immobilization of enzymes.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Biochemical and molecular modeling analysis of the ability of two p-aminobenzamidine-based sorbents to selectively purify serine proteases (fibrinogenases) from snake venoms.
De-Simone S G, et al.
Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 822(1-2), 1-9 (2005)
In vitro blood compatibility of polymeric biomaterials through covalent immobilization of an amidine derivative.
Gouzy M F, et al.
Biomaterials, 25(17), 3493-3501 (2004)
A L Nguyen et al.
Biotechnology and bioengineering, 34(9), 1186-1190 (1989-11-01)
Reactive polymers have been prepared by copolymeriz-ing N-isopropyl acrylamide (NIPAM) with N-acryloxy-succinimide (NASI) or glycidyl methacrylate (GMA). The amino groups of ligands could react with the residues of NASI or GMA and the polymers could be precipitated by temperature and/or
A L Nguyen et al.
Enzyme and microbial technology, 12(9), 663-668 (1990-09-01)
A reactive water-soluble polymer was synthesized by copolymerizing N-isopropylacrylamide and glycidyl acrylate. The reactive polymer could react with the amino groups of enzymes/proteins or other ligands to form an affinity polymer. As a model, the reactive polymer was allowed to
Specific adsorption of serine proteases on coated silica beads substituted with amidine derivatives.
S Khamlichi et al.
Journal of chromatography, 510, 123-132 (1990-06-27)
Amidine derivatives interact with serine proteases, the inhibition being due to interactions between amidine functions and the active sites of the enzymes. Five different types of amidine (substituted or unsubstituted) were coupled to coated silica beads, which had previously been
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej