D9568
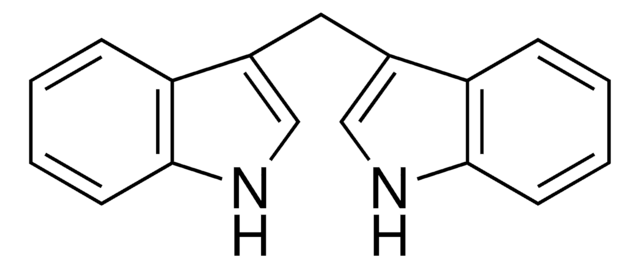
3,3′-Diindolylmethane
≥98% (HPLC)
Sinônimo(s):
3,3′-Bisindolylmethane, DIM
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥98% (HPLC)
Formulário
powder
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
C(c1c[nH]c2ccccc12)c3c[nH]c4ccccc34
InChI
1S/C17H14N2/c1-3-7-16-14(5-1)12(10-18-16)9-13-11-19-17-8-4-2-6-15(13)17/h1-8,10-11,18-19H,9H2
chave InChI
VFTRKSBEFQDZKX-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- 3,3′-Diindolylmethane inhibits Th17 cell differentiation via impairing IRF-7-mediated plasmacytoid dendritic cell activation in imiquimod-induced psoriasis mice.: The research indicates that 3,3′-Diindolylmethane can effectively inhibit Th17 cell differentiation, offering a potential therapeutic approach for treating psoriasis by targeting plasmacytoid dendritic cell pathways (Rasool et al., 2024).
- Protective effect of diindolylmethane-enriched dietary cabbage against doxorubicin-induced cardiotoxicity in mice.: This study highlights the cardioprotective effects of a diindolylmethane-enriched diet in mice, offering a dietary approach to mitigate the cardiotoxic effects of doxorubicin, a common chemotherapeutic agent (Natesh et al., 2024).
- Nanoformulated 3′-diindolylmethane modulates apoptosis, migration, and angiogenesis in breast cancer cells.: The investigation into nanoformulated 3′-diindolylmethane shows it significantly influences apoptosis, migration, and angiogenesis, suggesting its utility in targeted cancer therapies (Harakeh et al., 2024).
- Design, synthesis, and biological evaluation of 3,3′-diindolylmethane N-linked glycoconjugate as a leishmanial topoisomerase IB inhibitor with reduced cytotoxicity.: Research presents a synthesized glycoconjugate of 3,3′-Diindolylmethane as an effective inhibitor of leishmanial topoisomerase IB, demonstrating reduced cytotoxicity and potential as a therapeutic agent (Kour et al., 2023).
Ações bioquímicas/fisiológicas
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica