PHR1854
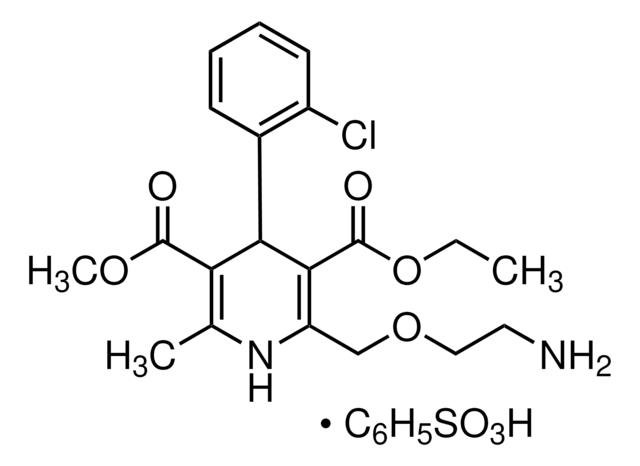
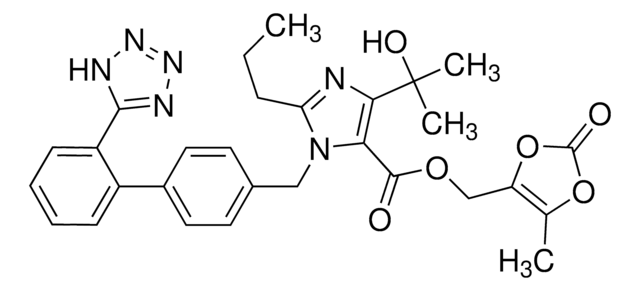
Candesartan Cilexetil
Pharmaceutical Secondary Standard; Certified Reference Material
Sinônimo(s):
Candesartan cilexetil, 2-ethoxy-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-Benzimidazole-7-carboxylic acid 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester, TCV 116, TCY 116
About This Item
Produtos recomendados
grau
certified reference material
pharmaceutical secondary standard
Nível de qualidade
Agency
traceable to Ph. Eur. Y0001388
traceable to USP 1087803
família API
candesartan
Formulário
powder
embalagem
pkg of 200 mg
aplicação(ões)
pharmaceutical
cadeia de caracteres SMILES
CCOc1nc2cccc(C(=O)OC(C)OC(=O)OC3CCCCC3)c2n1Cc4ccc(cc4)-c5ccccc5-c6nnn[nH]6
InChI
1S/C33H34N6O6/c1-3-42-32-34-28-15-9-14-27(31(40)43-21(2)44-33(41)45-24-10-5-4-6-11-24)29(28)39(32)20-22-16-18-23(19-17-22)25-12-7-8-13-26(25)30-35-37-38-36-30/h7-9,12-19,21,24H,3-6,10-11,20H2,1-2H3,(H,35,36,37,38)
chave InChI
GHOSNRCGJFBJIB-UHFFFAOYSA-N
Informações sobre genes
human ... AGTR1(185)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Candesartan cilexetil is an angiotensin II receptor antagonist used as a prodrug in the treatment of hypertension.
Aplicação
- Determination of candesartan cilexetil in tablet formulations by a UV/fluorescence spectrophotometric method
- Study of the release of candesartan cilexetil in tablet form by reversed-phase high-performance liquid chromatography (RP-HPLC)
- Simultaneous estimation of candesartan cilexetil and hydrochlorothiazide in pharmaceutical preparations using liquid chromatography in combination with photodiode array detector (DAD) and evaporative light scattering detector (ELSD)
- Spectroflourimetric determination of four angiotensin II receptor antagonists (AIIRA’s) in their pure form as well as pharmaceutical formulations
Ações bioquímicas/fisiológicas
Nota de análise
Nota de rodapé
Produtos recomendados
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Repr. 1B - STOT RE 2 Oral
Órgãos-alvo
Kidney,Blood
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica