P23954
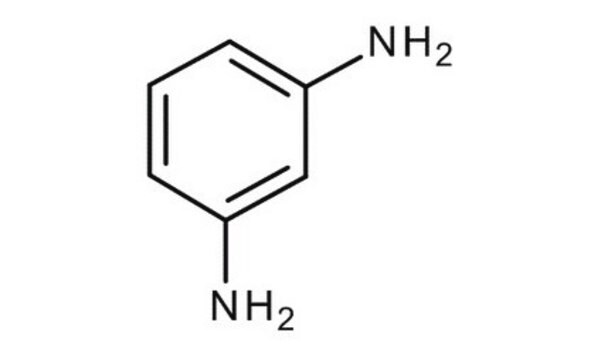
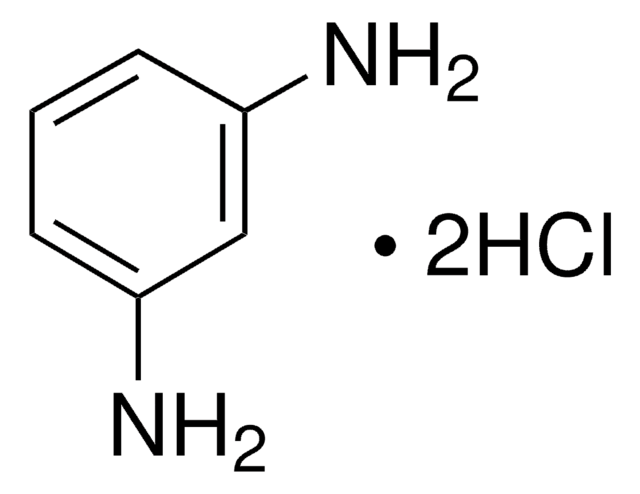
m-Phenylenediamine
flakes, 99%
Sinônimo(s):
1,3-Benzenediamine, 1,3-Diaminobenzene, 1,3-Phenylenediamine, MPDA
About This Item
Produtos recomendados
densidade de vapor
3.7 (vs air)
Nível de qualidade
pressão de vapor
0.62 mmHg ( 100 °C)
Ensaio
99%
Formulário
flakes
temperatura de autoignição
1040 °F
p.e.
282-284 °C
pf
64-66 °C
cadeia de caracteres SMILES
Nc1cccc(N)c1
InChI
1S/C6H8N2/c7-5-2-1-3-6(8)4-5/h1-4H,7-8H2
chave InChI
WZCQRUWWHSTZEM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- intrinsically electrically semiconducting microparticles of semiladder poly(m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) structures
- extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite, used as sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls
- series of terpolymers, via chemical oxidative polymerization
- thin film composite (TFC) membranes based polyamide
- TFC reverse osmosis (RO) membranes
Outras notas
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Muta. 2 - Skin Sens. 1
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Global Trade Item Number
| SKU | GTIN |
|---|---|
| P23954-25G | |
| P23954-500G | 4061834363026 |
| P23954-100G | 4061834363019 |
| P23954-15KG | 4061832768922 |
| P23954-1KG | 4061826220320 |
| P23954-5G | 4061834363033 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica