902586
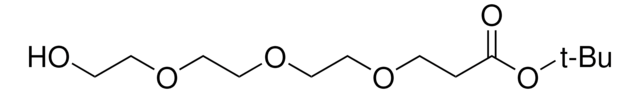
Hydroxy-PEG4-t-butyl ester
Sinônimo(s):
tert-Butyl-1-hydroxy-3,6,9,12-tetraoxapentadecan-15-oate, HO-PEG4-CO-OtBu
About This Item
Produtos recomendados
Ensaio
≥95%
forma
liquid
adequação da reação
reagent type: cross-linking reagent
índice de refração
n/D 1.4492
densidade
1.04746 g/mL
grupo funcional
ester
hydroxyl
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
O=C(OC(C)(C)C)CCOCCOCCOCCOCCO
InChI
1S/C15H30O7/c1-15(2,3)22-14(17)4-6-18-8-10-20-12-13-21-11-9-19-7-5-16/h16H,4-13H2,1-3H3
chave InChI
FJRDXEGYAVAMLB-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
Outras notas
Informações legais
produto relacionado
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica