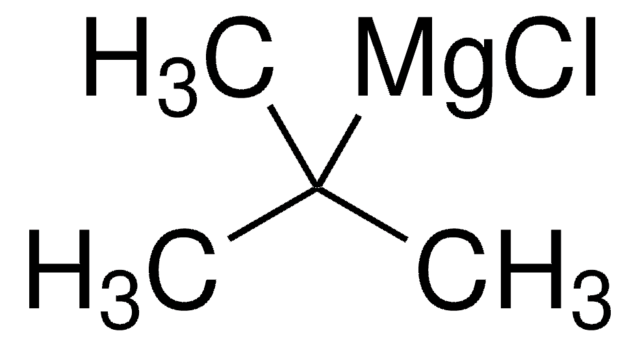
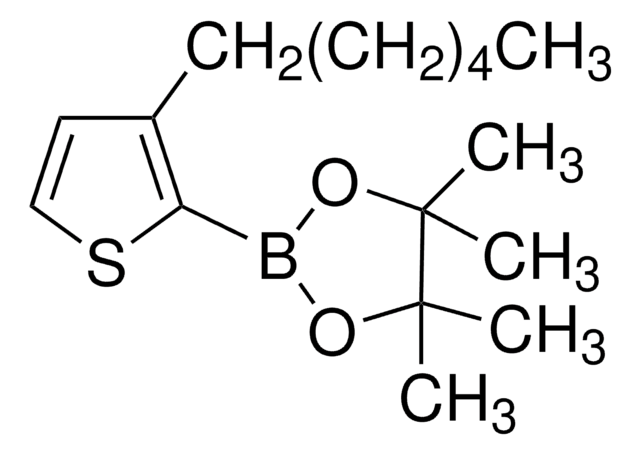
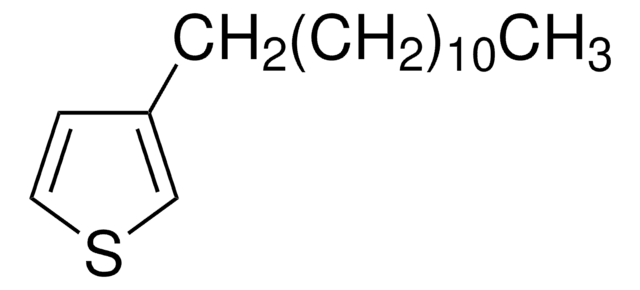456373
2,5-Dibromo-3-hexylthiophene
97%
Sinônimo(s):
2,5-Dibromo-3-hex-1-ylthiophene
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C10H14Br2S
Número CAS:
Peso molecular:
326.09
Número MDL:
Código UNSPSC:
12352103
ID de substância PubChem:
NACRES:
NA.23
Produtos recomendados
Nível de qualidade
Ensaio
97%
índice de refração
n20/D 1.557 (lit.)
densidade
1.521 g/mL at 25 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CCCCCCc1cc(Br)sc1Br
InChI
1S/C10H14Br2S/c1-2-3-4-5-6-8-7-9(11)13-10(8)12/h7H,2-6H2,1H3
chave InChI
NSYFIAVPXHGRSH-UHFFFAOYSA-N
Descrição geral
2,5-Dibromo-3-hexylthiophene is a 2,5 coupled conductive polymer with conjugated polythiophene based system, which has a controllable band gap.
Aplicação
Conducting polymer precursor.
It can be used as a monomer with 5,5′-dibromo-3,3′-dihexyl-2,2′-bithiophene, which can synthesize regioregular-P3HT-regiosymmetric-P3HT (a diblock polymer) for organic electronics based applications. It can also be used in the synthesis of P3HT, which can be potentially used for photocatalytic applications.
Informações legais
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Charge Transport in Conjugated Polymers with Pendent Stable Radical Groups.
Zhang Y, et al.
Chemistry of Materials (2018)
Paulina Powroznik et al.
Sensors (Basel, Switzerland), 20(2) (2020-01-19)
In the present work, we report the use of regioregular poly(3-hexyltiophene) polymer (RR-P3HT) as a potential light-activated material for sensing the chemical nerve agent simulant dimethyl methylphosphonate (DMMP). The electrical response of thick films of RR-P3HT, deposited by spray-coating method
Tomasz Jarosz et al.
Polymers, 11(2) (2019-04-10)
A type of graft copolymer based on polysiloxane and regioregular poly(3-hexylthiophene) (P3HT) has been synthesised and its properties have been studied alongside those of its parent conjugated polymer-regioregular P3HT. Electrochemical analysis has revealed more significant changes in conformation of the
Xiaoyu Li et al.
Nature communications, 8, 15909-15909 (2017-06-27)
Micelles formed by the self-assembly of block copolymers in selective solvents have attracted widespread attention and have uses in a wide variety of fields, whereas applications based on their electronic properties are virtually unexplored. Herein we describe studies of solution-processable
Kinga Kepska et al.
Chemicke zvesti, 72(1), 251-259 (2018-01-26)
The first comprehensive spectroelectrochemical account of the behaviour of regioregular (RR-P3HT) and statistical (ST-P3HT) poly(3-hexylthiophenes) in solution is presented, in contrast to the many reports dealing with P3HT films merely deposited from solution. The conducted experiments revealed that the two
Artigos
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica


![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)