Synthetic Strategy for Large Scale Production of Oligothiophenes
Kanth Josyula, Mitesh Patel, Kaushik Patel, Scott Batcheller
Introduction
Oligothiophenes and their functional derivatives are used in a number of high-technology applications including OLEDs, OFETs and OPVs, where the ability to functionalize the oligothiophene backbone allows for the fine-tuning of the electronic properties.1
These functional oligothiophenes can be synthesized from synthetic intermediates via a number of different reactions. Suzuki coupling2 has proven to be particularly advantageous in the large scale production of oligothiophenes. Among the advantages of Suzuki coupling are predictable scaling reactions, less-toxic reagents, simple removal of metallic impurities and the ability to further purify products by sublimation. In addition, coupling building blocks are commercially available, allowing for the simple synthesis of any required oligothiophene.
General Synthetic Strategy for Large Scale Production of Oligothiophenes
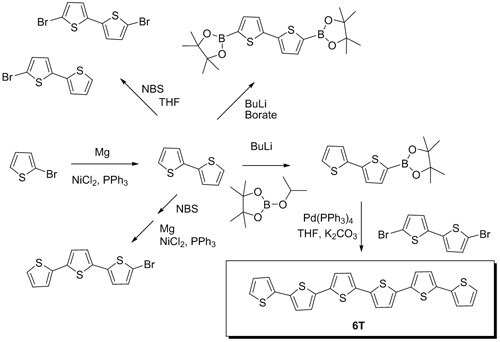
Figure 1.illustrates different synthetic pathways to oligothiophene building blocks, and ultimately to functional oligothiophenes via Suzuki Coupling.
Oligothiophene Constructor
Functional oligiothiophenes can be synthesized by reacting boronic acid and bromide intermediates in the presence of a palladium catalyst and a base as part of a Suzuki Coupling reaction.

Figure 2.Oligothiophene Constructor
Organic Electronic Materials
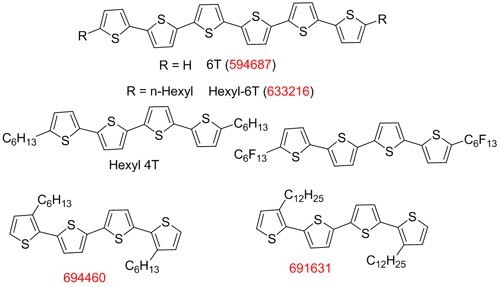
Figure 3.Illustrates a representation of functional oligothiophenes synthesized via Suzuki Coupling.
References
To continue reading please sign in or create an account.
Don't Have An Account?