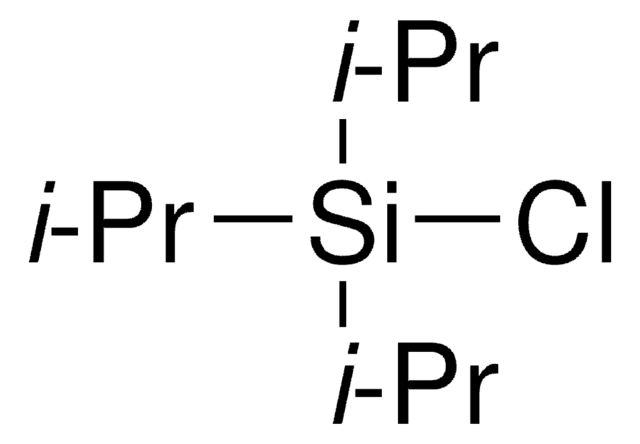About This Item
Fórmula empírica (Notação de Hill):
C13H25NSi
Número CAS:
Peso molecular:
223.43
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
95%
Formulário
liquid
índice de refração
n20/D 1.492 (lit.)
p.e.
78 °C/0.4 mmHg (lit.)
densidade
0.904 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
CC(C)[Si](C(C)C)(C(C)C)n1cccc1
InChI
1S/C13H25NSi/c1-11(2)15(12(3)4,13(5)6)14-9-7-8-10-14/h7-13H,1-6H3
chave InChI
FBQURXLBJJNDBX-UHFFFAOYSA-N
Descrição geral
1-(Triisopropylsilyl)pyrrole (TISP), a heterocyclic building block, is a pyrrole derivative. TISP has been reported to generate pyrrolic cation radicals during cyclovoltammetric studies, via electroreduction. It participates in various electrophilic substitution reactions specifically at β-position, via reaction with various electrophilic reagents (Br+, I+,NO2+,etc).
Aplicação
1-(Triisopropylsilyl)pyrrole may be employed as reagent in perfluoroalkylation and Vilsmeier formylation reactions. It may be used in the preparation of:
- ethyl 2-(2,4-dinitrophenylhydrazono]-3-[ 1-(triisopropylsily1)-pyrrol-2-yflpropanoate
- heterocyclic base, 3-nitropyrrole
- 3-nitropyrrole, required for the synthesis of 1 -(2′-deoxy-β-D-ribofuranosyl)-3-nitropyrrole
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
224.6 °F - closed cup
Ponto de fulgor (°C)
107 °C - closed cup
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
N-(triisopropylsilyl) pyrrole. A progenitor" par excellence" of 3-substituted pyrroles.
Bray BL, et al.
The Journal of Organic Chemistry, 55(26), 6317-6328 (1990)
Daniel A Harki et al.
Biochemistry, 41(29), 9026-9033 (2002-07-18)
Synthetic small molecules that promote viral mutagenesis represent a promising new class of antiviral therapeutics. Ribavirin is a broad-spectrum antiviral nucleoside whose antiviral mechanism against RNA viruses likely reflects the ability of this compound to introduce mutations into the viral
Observation of the cation radicals of pyrrole and of some substituted pyrroles in fast-scan cyclic voltammetry. Standard potentials and lifetimes.
Andrieux CP, et al.
Journal of the American Chemical Society, 112(6), 2439-2440 (1990)
Reaction of pyrroles with ethyl 2-nitroso-and 2-azo-propenoates, and with ethyl cyanoformate N-oxide: a comparison of the reaction pathways.
Gilchrist TL and Lemos A.
Journal of the Chemical Society. Perkin Transactions 1, 13, 1391-1395 (1993)
Synthesis, Structure, and Deoxyribonucleic Acid Sequencing with a Universal Nucleoside: 1-(2'-Deoxy-. beta.-D-ribofuranosyl)-3-nitropyrrole.
Bergstrom DE, et al.
Journal of the American Chemical Society, 117(4), Synthesis-Synthesis (1999)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica