425834
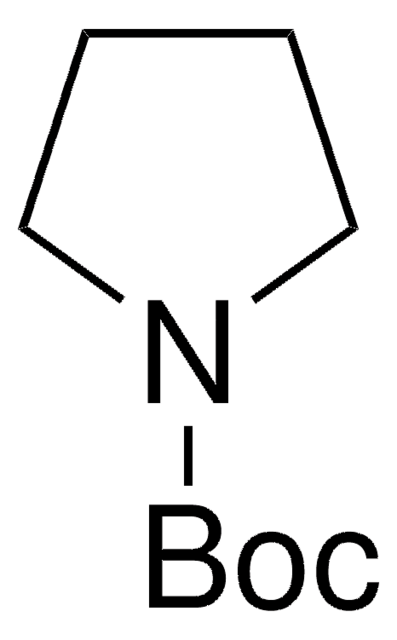
N-Boc-pyrrole
98%
Sinônimo(s):
tert-Butyl 1-pyrrolecarboxylate
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C9H13NO2
Número CAS:
Peso molecular:
167.21
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
forma
liquid
índice de refração
n20/D 1.4685 (lit.)
pb
91-92 °C/20 mmHg (lit.)
densidade
1 g/mL at 25 °C (lit.)
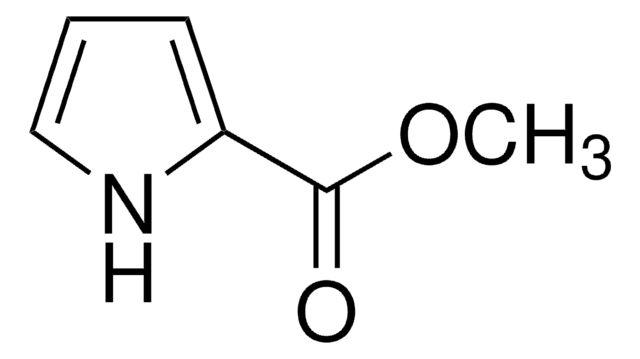
cadeia de caracteres SMILES
CC(C)(C)OC(=O)n1cccc1
InChI
1S/C9H13NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-7H,1-3H3
chave InChI
IZPYBIJFRFWRPR-UHFFFAOYSA-N
Descrição geral
N-Boc-pyrrole is an N-protected pyrrole. It undergoes Diels–Alder reaction with enantiomerically pure allene-1,3-dicarboxylates to form endo-adducts with retention in configurations at two newly generated stereogenic centers. It also undergoes cyclopropanation with methyl phenyldiazoacetate to form both monocyclopropane and dicyclopropane. Its Ir-catalyzed C-H borylation followed by cross coupling with 3-chlorothiophene to form biheterocycle has been reported.
Aplicação
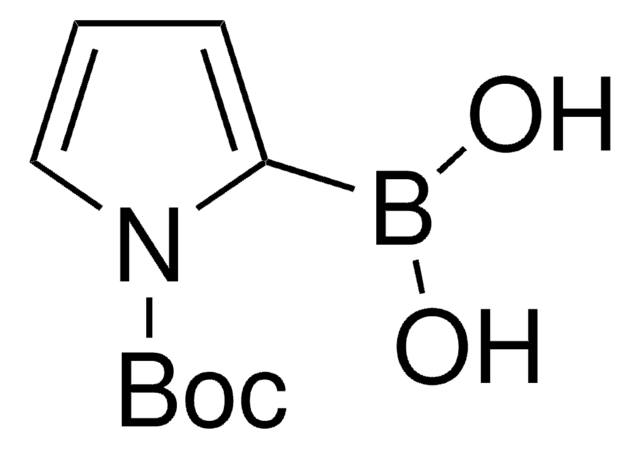
N-Boc-pyrrole was used in the synthesis of 1-(tert-butoxycarbonyl)-1H-pyrrol-2-ylboronic acid by treating with n-BuLi and subsequent reaction with trimethyl borate.
It may be used as starting material in the synthesis of the following:
It may be used as starting material in the synthesis of the following:
- tropane drivatives
- N-boc-2-(4-methoxyphenyl)pyrrole
- N-boc-pyrrol-2-ylboronic acid
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
167.0 °F - closed cup
Ponto de fulgor (°C)
75 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Synthetic approaches to enantiomerically pure 8-azabicyclo [3.2. 1] octane derivatives.
Pollini GP, et al.
Chemical Reviews, 106(6), 2434-2454 (2006)
Huw M L Davies et al.
Chemical Society reviews, 38(11), 3061-3071 (2009-10-23)
The metal catalyzed reactions of diazo compounds have been broadly used in organic synthesis. The resulting metal-carbenoid intermediates are capable of undergoing a range of unconventional reactions, and due to their high energy, they are ideal for initiating cascade sequences
Recent progress in the synthesis of five-membered heterocycle boronic acids and esters.
Primas N, et al.
Tetrahedron, 66(41), 8121-8136 (2010)
Nikola Basarić et al.
Organic & biomolecular chemistry, 3(15), 2755-2761 (2005-07-21)
Two fluorescent off-on Ca2+ indicators based on APTRA (o-aminophenol-N,N,O-triacetic acid) as low-affinity ligand for Ca2+ and BODIPY(4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) as a fluorophore were synthesized. The new BODIPY-APTRA compounds absorb in the visible spectrum, with absorption maxima from 505 nm to 570 nm
CuO/SiO2 as a simple, effective and recoverable catalyst for alkylation of indole derivatives with diazo compounds.
Fraile JM, et al.
Organic & Biomolecular Chemistry, 11(26), 4327-4332 (2013)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica