281077
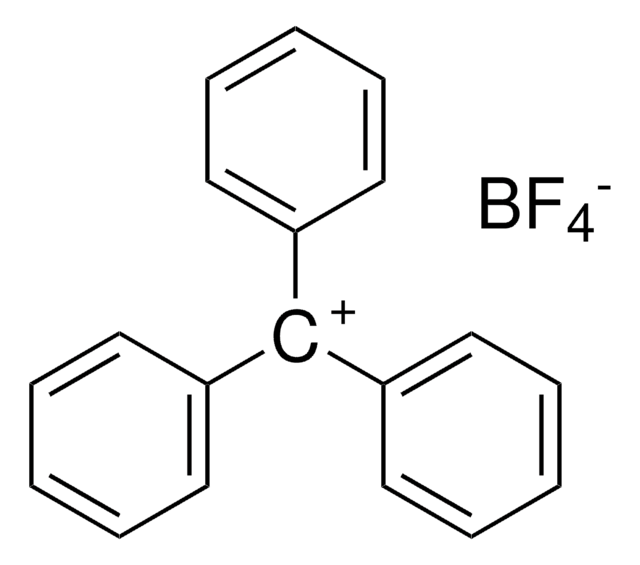
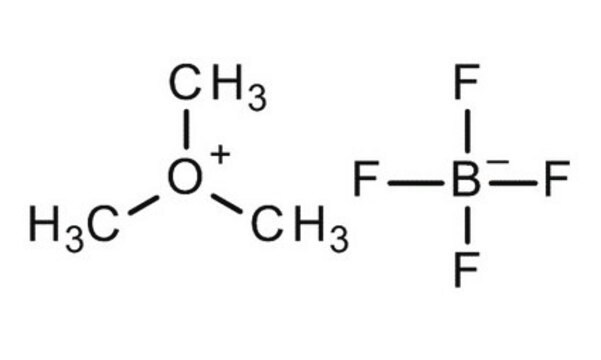
Trimethyloxonium tetrafluoroborate
95%
Sinônimo(s):
Trimethyloxonium fluoroborate
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
(CH3)3O(BF4)
Número CAS:
Peso molecular:
147.91
Beilstein:
3597303
Número CE:
Número MDL:
Código UNSPSC:
12352107
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
95%
Formulário
solid
grupo funcional
ether
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
C[O+](C)C.F[B-](F)(F)F
InChI
1S/C3H9O.BF4/c1-4(2)3;2-1(3,4)5/h1-3H3;/q+1;-1
chave InChI
CZVZBKHWOFJNCR-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Trimethyloxonium tetrafluoroborate can be used as a methylating agent for the methylation of hydroxyl/carboxyl functional groups. It is capable of methylating polyfunctional carboxylic acids. It is also used as a catalyst for the polymerization of cyclic sulfides and ethers.
Aplicação
Reagent for the methylation of hydroxyl groups recently used in a complex, multistep synthesis directed towards spirastrellolide, a marine natural product.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Skin Corr. 1B
Perigos de suplementos
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Toward the total synthesis of spirastrellolide A. Part 2: Conquest of the northern hemisphere.
Alois Fürstner et al.
Angewandte Chemie (International ed. in English), 45(33), 5510-5515 (2006-08-15)
H M Liebich et al.
Journal of chromatography. A, 843(1-2), 237-245 (1999-07-10)
Trimethyloxonium tetrafluoroborate (TMO) is applied as derivatising reagent to transform urinary organic acids into their methyl esters. The method is suggested as an alternative to the use of diazomethane which is carcinogenic and explosive. In contrast to other methods avoiding
Marco Pacenti et al.
Biomedical chromatography : BMC, 22(10), 1155-1163 (2008-05-29)
A method for the determination of the organic acids directly in the urine employing derivatization with trimethyloxonium tetrafluoroborate as a methylating agent and sequential extraction by head space and direct immersion/solid phase microextraction is reported. Furoic acid, hippuric acid, methylhippuric
S Chericoni et al.
Journal of analytical toxicology, 35(4), 193-198 (2011-04-26)
The present work describes the validation of a novel aqueous in situ derivatization procedure with trimethyloxonium tetrafluoroborate (TMO) as methylating agent for the simultaneous, quantitative analysis of Δ(9)-tetrahydrocannabinol (THC) and 11-nor-Δ(9)-tetrahydrocannabinol carboxylic acid (THC-COOH) in human urine. The derivatizing agent
H M Liebich et al.
Journal of chromatography. B, Biomedical sciences and applications, 713(2), 427-432 (1998-09-24)
We developed a new sample preparation method for profiling organic acids in urine by GC or GC-MS. The method includes derivatisation of the organic acids directly in the aqueous urine using trimethyloxonium tetrafluoroborate as a methylating agent, extraction of the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica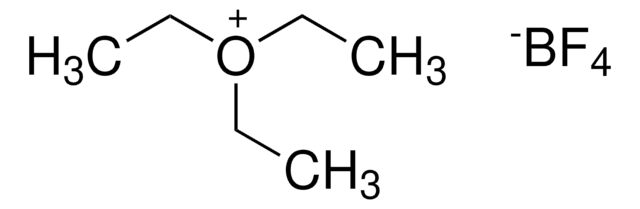


![1,8-Diazabiciclo[5,4,0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)