227439
Lawesson reagent
97%
Sinônimo(s):
2,4-Bis(4-methoxyphenyl)-2,4-dithioxo-1,3,2,4-dithiadiphosphetane, 2,4-Bis-(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane 2,4-disulfide, 4-Methoxyphenylthiophosphoric cyclic di(thioanhydride), LR
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
powder
pf
228-230 °C (lit.)
cadeia de caracteres SMILES
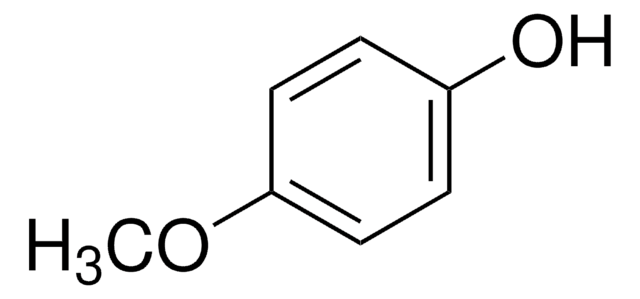
COc1ccc(cc1)P2(=S)SP(=S)(S2)c3ccc(OC)cc3
InChI
1S/C14H14O2P2S4/c1-15-11-3-7-13(8-4-11)17(19)21-18(20,22-17)14-9-5-12(16-2)6-10-14/h3-10H,1-2H3
chave InChI
CFHGBZLNZZVTAY-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- Oxthiaphosphinine-3-sulfide derivatives by the reaction with Mannich bases of β-naphthol and 8-hydroxyquinoline.
- 1,3,5,2-Trithiaphosphinane-2-sulfide derivatives by reacting with benzaldehyde in the presence of trialkyl phosphite.
- 2,4,6-Triphenyl-1,3,5-trithiane from benzaldehyde and ethyl acrylate.
- 9-Benzanthronethione by thionation of 9-benzanthone oxime.
- 1,2,4-Trithiolane from 2,2,4,4-tetramethyl-3-thioxocyclobutanone S-oxide.
- Sulfur derivatives of triterpenic oxo compounds.
- Tropothione in situ at room temperature and to trap it with dieneophiles.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Water-react 2
Perigos de suplementos
Código de classe de armazenamento
4.3 - Hazardous materials which set free flammable gases upon contact with water
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica