254037
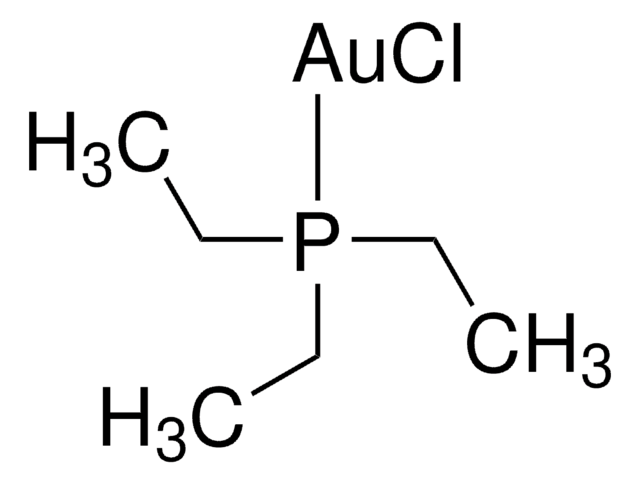
Chloro(triphenylphosphine)gold(I)
≥99.9% trace metals basis
Sinônimo(s):
(Ph3P)AuCl, Triphenylphosphinegold(I) chloride
About This Item
Produtos recomendados
Ensaio
≥99.9% trace metals basis
Formulário
solid
adequação da reação
core: gold
reagent type: catalyst
cadeia de caracteres SMILES
Cl[Au].c1ccc(cc1)P(c2ccccc2)c3ccccc3
InChI
1S/C18H15P.Au.ClH/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;/h1-15H;;1H/q;+1;/p-1
chave InChI
IFPWCRBNZXUWGC-UHFFFAOYSA-M
Aplicação
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
The importance of selectively fluorinating compounds in medicinal chemistry, biology, and organic synthesis is well appreciated and provides a major impetus to the discovery of new and mild fluorinating agents that can operate safely and efficiently.
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![Chloro[tris(para-trifluoromethylphenyl)phosphine]gold(I) 99%](/deepweb/assets/sigmaaldrich/product/structures/250/453/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e/640/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e.png)

![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)
gold(I) (2:1) toluene adduct](/deepweb/assets/sigmaaldrich/product/structures/104/897/81ee3e56-c988-4d0f-9614-1269b470316d/640/81ee3e56-c988-4d0f-9614-1269b470316d.png)

![Chloro[2-dicyclohexyl(2′,4′,6′-trisopropylbiphenyl)phosphine]gold(I)](/deepweb/assets/sigmaaldrich/product/structures/253/590/7ea3a0c9-1b4c-4e68-8fa0-5e764f73519a/640/7ea3a0c9-1b4c-4e68-8fa0-5e764f73519a.png)
![Chloro[tris(2,4-di-tert-butylphenyl)phosphite]gold](/deepweb/assets/sigmaaldrich/product/structures/386/294/6df0db46-002b-4599-ad6c-451c419a3fc5/640/6df0db46-002b-4599-ad6c-451c419a3fc5.png)
![(Acetonitrile)[(2-biphenyl)di-tert-butylphosphine]gold(I) hexafluoroantimonate](/deepweb/assets/sigmaaldrich/product/structures/216/222/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9/640/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9.png)