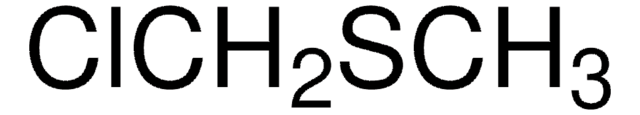About This Item
Fórmula linear:
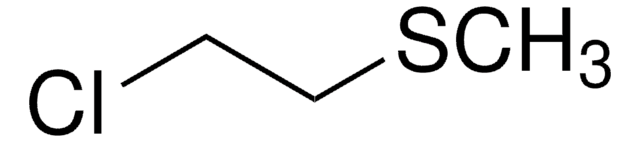
ClCH2CH2SC2H5
Número CAS:
Peso molecular:
124.63
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
Formulário
liquid
índice de refração
n20/D 1.4885 (lit.)
p.e.
156-157 °C (lit.)
densidade
1.07 g/mL at 25 °C (lit.)
grupo funcional
chloro
thioether
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CCSCCCl
InChI
1S/C4H9ClS/c1-2-6-4-3-5/h2-4H2,1H3
chave InChI
GBNVXYXIRHSYEG-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
2-Chloroethyl ethyl sulfide is a monofunctional analog of sulfur mustard (SM; 2,2′-dichloro diethyl sulfide). The mass diffusivity of 2-chloroethyl ethyl sulfide, a chemical warfare agent simulant, was studied.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 1A - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
125.6 °F - closed cup
Ponto de fulgor (°C)
52 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Adrienne T Black et al.
Toxicology and applied pharmacology, 249(2), 178-187 (2010-09-16)
Sulfur mustard is a potent vesicant that induces inflammation, edema and blistering following dermal exposure. To assess molecular mechanisms mediating these responses, we analyzed the effects of the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide, on EpiDerm-FT™, a commercially available
Heidi C O'Neill et al.
Free radical biology & medicine, 48(9), 1188-1196 (2010-02-09)
Sulfur mustard (bis-2-(chloroethyl) sulfide; SM) is a highly reactive vesicating and alkylating chemical warfare agent. A SM analog, 2-chloroethyl ethyl sulfide (CEES), has been utilized to elucidate mechanisms of toxicity and as a screen for therapeutics. Previous studies with SM
Tabea Zubel et al.
Archives of toxicology, 93(1), 61-79 (2018-10-17)
Despite its worldwide ban, the warfare agent sulfur mustard (SM) still represents a realistic threat, due to potential release in terroristic attacks and asymmetric conflicts. Therefore, the rigorous and quantitative detection of SM exposure is crucial for diagnosis, health risk
Swetha Inturi et al.
Free radical biology & medicine, 51(12), 2272-2280 (2011-09-17)
Employing mouse skin epidermal JB6 cells and dermal fibroblasts, here we examined the mechanisms of DNA damage by 2-chloroethyl ethyl sulfide (CEES), a monofunctional analog of sulfur mustard (SM). CEES exposure caused H2A.X and p53 phosphorylation as well as p53
Joshua P Gray et al.
Toxicology and applied pharmacology, 247(2), 76-82 (2010-06-22)
Inhalation of vesicants including sulfur mustard can cause significant damage to the upper airways. This is the result of vesicant-induced modifications of proteins important in maintaining the integrity of the lung. Cytochrome P450s are the major enzymes in the lung
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica