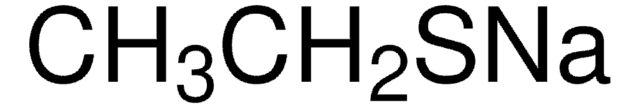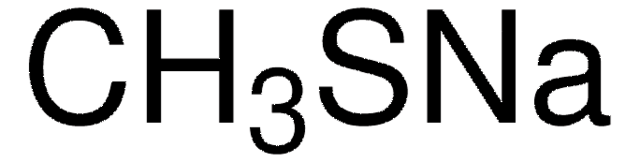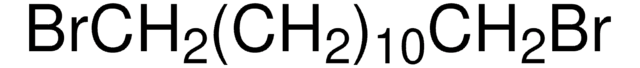241776
Potassium thioacetate
98%
Sinônimo(s):
Ethanethioic acid potassium salt, Thioacetic acid potassium salt, Thiolacetic acid potassium salt
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
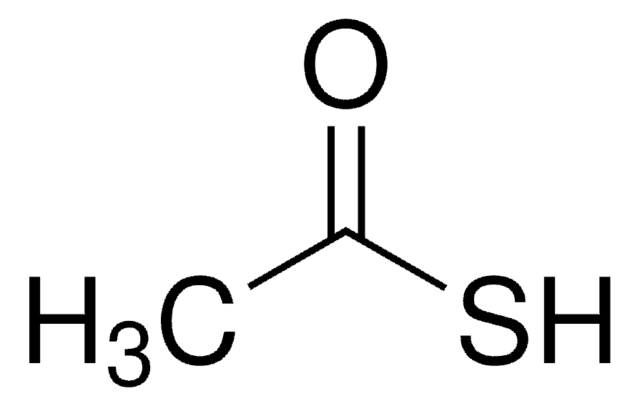
CH3COSK
Número CAS:
Peso molecular:
114.21
Beilstein:
4428862
Número CE:
Número MDL:
Código UNSPSC:
12352302
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
grau
for analytical purposes
Ensaio
98%
Formulário
solid
pf
173-176 °C (lit.)
cadeia de caracteres SMILES
[K+].CC([S-])=O
InChI
1S/C2H4OS.K/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1
chave InChI
AFNBMGLGYSGFEZ-UHFFFAOYSA-M
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Potassium thioacetate is a sulfur source in sulfuration reactions and is used as a reagent in nucleophilic substitution and vinylic substitution reactions.
Aplicação
Palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives.
Reagent in the preparation of thiols from halides.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Subal Dey et al.
Inorganic chemistry, 57(10), 5939-5947 (2018-05-02)
Reduction of CO2 holds the key to solving two major challenges taunting the society-clean energy and clean environment. There is an urgent need for the development of efficient non-noble metal-based catalysts that can reduce CO2 selectively and efficiently. Unfortunately, activation
Yang Wang et al.
Polymers, 11(6) (2019-06-13)
Photodynamic therapy (PDT) as a non-aggressive therapy with fewer side effects has unique advantages over traditional treatments. However, PDT still has certain limitations in clinical applications, mainly because most photosensitizers utilized in PDT are hydrophobic compounds, which will self-aggregate in
Tetrahedron Letters, 48, 3033-3033 (2007)
Christina Wedemeyer-Exl et al.
Organic & biomolecular chemistry, 5(13), 2119-2128 (2007-06-22)
The thiol-dependent methylation of heptamethyl cob(II)yrinate 8r with methyl iodide and methyl tosylate was explored under a variety of conditions. The interaction of the heptamethyl cob(II)yrinate with a variety of thiols was monitored prior to the addition of the methylating
Ning Shangguan et al.
Journal of the American Chemical Society, 125(26), 7754-7755 (2003-06-26)
A new amide synthesis strategy based on a fundamental mechanistic revision of the reaction of thio acids and organic azides is presented. The data demonstrate that amines are not formed as intermediates in this reaction. Alternative mechanisms proceeding through a
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica