T30805
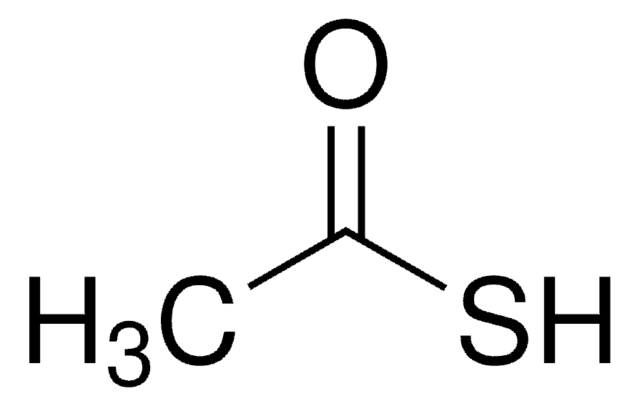
Thioacetic acid
96%
Sinônimo(s):
TAA, TMA, Thiacetic acid
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
96%
índice de refração
n20/D 1.465 (lit.)
pb
88-91.5 °C (lit.)
densidade
1.065 g/mL at 25 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CC(S)=O
InChI
1S/C2H4OS/c1-2(3)4/h1H3,(H,3,4)
chave InChI
DUYAAUVXQSMXQP-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Enantioselective addition to nitroalkenes to form chiral 1,2-aminothiol derivatives in the presence of a novel sulfinyl urea organocatalyst. This method has been successfully employed in the synthesis of antifungal drug, sulconazole.
- Asymmetric Michael addition reaction with chalcones in the presence of a bifunctional amine thiourea catalyst to form synthetically useful thioesters.
- Asymmetric 1,6-conjugate addition with para-quinone methides in the presence of a chiral phosphoric acid catalyst to form chiral sulfur-containing diphenylmethane-type compounds.
- Conjugate addition to methacrylamides with chiral trans-2,5-disubstituted pyrrolidine auxiliaries to form chiral β-mercaptocarboxylic acid derivatives.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Sens. 1
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
64.4 °F - closed cup
Ponto de fulgor (°C)
18 °C - closed cup
Equipamento de proteção individual
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica