105449
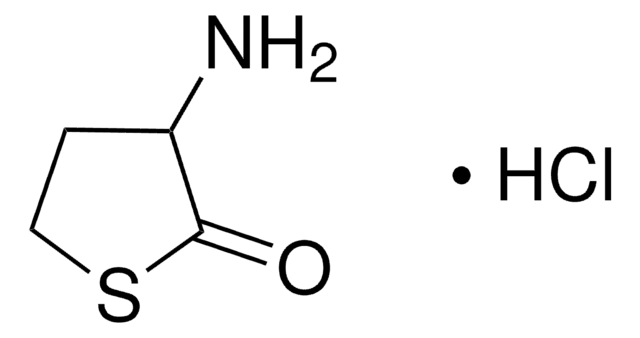
γ-Thiobutyrolactone
98%
Sinônimo(s):
4-Butyrothiolactone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C4H6OS
Número CAS:
Peso molecular:
102.15
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
índice de refração
n20/D 1.523 (lit.)
p.e.
39-40 °C/1 mmHg (lit.)
solubilidade
THF: soluble
densidade
1.18 g/mL at 25 °C (lit.)
grupo funcional
thioester
cadeia de caracteres SMILES
O=C1CCCS1
InChI
1S/C4H6OS/c5-4-2-1-3-6-4/h1-3H2
chave InChI
KMSNYNIWEORQDJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
γ-Thiobutyrolactone undergoes copolymerization with glycidyl phenyl ether to form poly(ester-alt-sulfide).
Aplicação
γ-Thiobutyrolactone was used to terminate the ring opening polymerization of ω-pentadecalactone to synthesize difunctional polyesters. γ-Thiobutyrolactone was used to study the mechanism of metabolism of sulphur containing heterocyclic compounds by lignin-degrading basidiomycete Coriolus versicolor.
Palavra indicadora
Warning
Frases de perigo
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
186.8 °F - closed cup
Ponto de fulgor (°C)
86 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Tiny droplets make a big splash.
Michael Eisenstein
Nature methods, 3(2), 71-71 (2006-02-14)
Jonathan Garel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(15), 4144-4152 (2006-02-03)
Homocysteine thiolactone (tHcy) is deemed a risk factor for cardiovascular diseases and strokes, presumably because it acylates the side chain of protein lysine residues ("N-homocysteinylation"), thereby causing protein damage and autoimmune responses. We analysed the kinetics of hydrolysis and aminolysis
Nishikubo et al.
Macromolecules, 31(15), 4746-4752 (1998-07-29)
Poly(ester-alt-sulfide) (polymer 1) was synthesized by the alternating copolymerization of glycidyl phenyl ether (GPE) with gamma-thiobutyrolactone (TBL) catalyzed by either quaternary onium salts or crown ether complexes. The copolymerization proceeded to produce polymer 1 with good yields in neat or
K D Holland et al.
Brain research, 615(1), 170-174 (1993-06-25)
Effects of alkyl-substituted gamma-butyrolactones and gamma-thiobutyrolactones on [35S]t-butylbicyclophosphorothionate (35S-TBPS) dissociation from the picrotoxinin receptor were studied. Unlike picrotoxinin, these lactones accelerated the dissociation rate of 35S-TBPS. Thus, previous reports that these lactones change the Kd but not the Bmax of
One-pot difunctionalization of poly (ω-pentadecalactone) with thiol-thiol or thiol-acrylate groups, catalyzed by Candida antarctica lipase B.
Takwa M, et al.
Macromolecular Rapid Communications, 27(22), 1932-1936 (2006)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica