56480
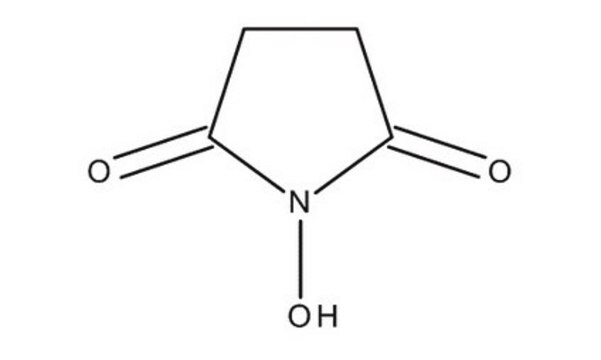
N-Hydroxysuccinimide
≥97.0% (T), for peptide synthesis
Synonym(s):
1-Hydroxy-2,5-pyrrolidinedione, HOSu, NHS
About This Item
Recommended Products
Product Name
N-Hydroxysuccinimide, purum, ≥97.0% (T)
grade
purum
Quality Level
assay
≥97.0% (T)
form
solid
reaction suitability
reaction type: Addition Reactions
mp
95-98 °C (lit.)
95-98 °C
application(s)
peptide synthesis
functional group
imide
SMILES string
ON1C(=O)CCC1=O
InChI
1S/C4H5NO3/c6-3-1-2-4(7)5(3)8/h8H,1-2H2
InChI key
NQTADLQHYWFPDB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- To synthesize N-succinimidyl 3-(2-pyridyldithio)-propionate, a heterobifunctional reagent useful for protein-protein conjugation and also to incorporate aliphatic thiols into proteins.
- To synthesize NHS esters of long-chain fatty acids.
- NHS can activate the phosphonic acid monolayers immobilized on titanium surface for binding with proteins.
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service