E6383
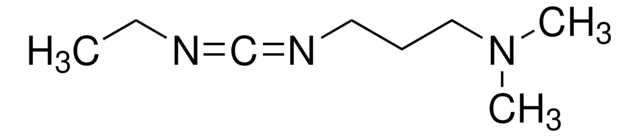
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
crystalline
Synonym(s):
EDC, N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride, EDAC, EDC hydrochloride, WSC hydrochloride
About This Item
Recommended Products
product name
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, crystalline
form
crystalline
Quality Level
reaction suitability
reagent type: cross-linking reagent
reaction type: Peptide Synthesis
color
white to off-white
mp
110-115 °C (lit.)
solubility
H2O: ≤100 mg/mL
application(s)
advanced drug delivery
general analytical
storage temp.
−20°C
SMILES string
Cl.CCN=C=NCCCN(C)C
InChI
1S/C8H17N3.ClH/c1-4-9-8-10-6-5-7-11(2)3;/h4-7H2,1-3H3;1H
InChI key
FPQQSJJWHUJYPU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Beyond peptides, EDC HCl extends its influence to the construction of immunogens, where it covalently attaches haptens (small immune-response eliciting molecules) to carrier proteins, playing an instrumental role in vaccine research. The versatility of EDAC HCl further unfolds in its ability to modify nucleic acids, allowing for the labeling of DNA and RNA through their 5′ phosphate groups. This facilitates the visualization, tracking, and analysis of these essential molecules, contributing to advancements in nucleic acid research.
Additionally, EDAC HCl serves as a biomolecule bridge by acting as a crosslinker, connecting amine-reactive NHS-esters of biomolecules to carboxyl groups. This technique proves valuable in protein conjugation, enabling the creation of hybrid molecules with novel properties and functions. The underlying mechanism of EDAC HCl involves its reaction with a carboxyl group, forming an unstable intermediate that actively seeks an amine partner. The delicate balance of this reaction underscores the importance of optimizing conditions for efficient conjugation. The assistance of N-hydroxysuccinimide (NHS) further enhances EDAC HCl′s capabilities, stabilizing the intermediate and enabling two-step conjugation procedures. This additional feature provides greater flexibility and control, particularly in dealing with complex biomolecules.
Application
- for the immobilisation of trypsin onto self-assembled monolayers (SAMs)
- as a component for the preparation of collagen matrices
- for the preparation of phosphoethanolamine(PEt)-conjugated sepharose
Biochem/physiol Actions
Features and Benefits
Other Notes
comparable product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
target_organs
Stomach,large intestine,lymph node
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide methiodide](/deepweb/assets/sigmaaldrich/product/structures/414/134/4eb9c126-d7f9-4e12-9e3a-95cb077824fd/640/4eb9c126-d7f9-4e12-9e3a-95cb077824fd.png)