333859
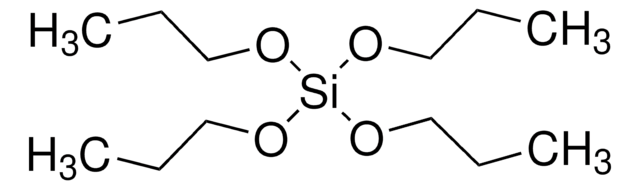
Tetraethyl orthosilicate
99.999% trace metals basis
Synonym(s):
Orthosilicic acid tetraethyl ester, Silicon tetraethoxide, TEOS, Tetraethoxysilane, Tetraethoxysilicon(IV), Tetraethyl silicate
About This Item
Recommended Products
vapor density
7.2 (vs air)
Quality Level
vapor pressure
<1 mmHg ( 20 °C)
assay
99.999% trace metals basis
form
liquid
refractive index
n20/D 1.382 (lit.)
bp
168 °C (lit.)
density
0.933 g/mL at 20 °C (lit.)
SMILES string
CCO[Si](OCC)(OCC)OCC
InChI
1S/C8H20O4Si/c1-5-9-13(10-6-2,11-7-3)12-8-4/h5-8H2,1-4H3
InChI key
BOTDANWDWHJENH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 3 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
113.0 °F - closed cup
flash_point_c
45 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
From Form to Function: Molding Porous Materials in Three Dimensions by Colloidal Crystal Templating
Research involving reactive silicone chemistry has focused on the production of pure silicon and hybrid materials, hydrosilylation, ring-opening and atom transfer polymerizations, polymerizations with controlled stereochemistry, and condensation reactions.
Mesoporous Oxides and Their Applications to Hydrogen Storage
Recent demand for electric and hybrid vehicles, coupled with a reduction in prices, has caused lithium-ion batteries (LIBs) to become an increasingly popular form of rechargeable battery technology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service