Key Documents
SML1779
Nigericin sodium salt
from Streptomyces hygroscopicus, ≥98% (HPLC), solution, polyether ionophore
Synonim(y):
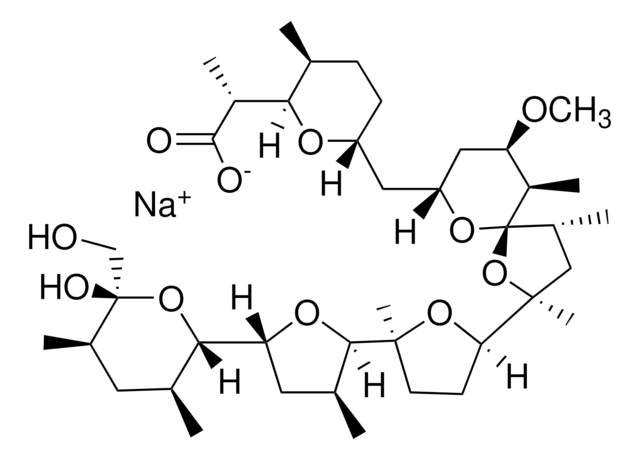
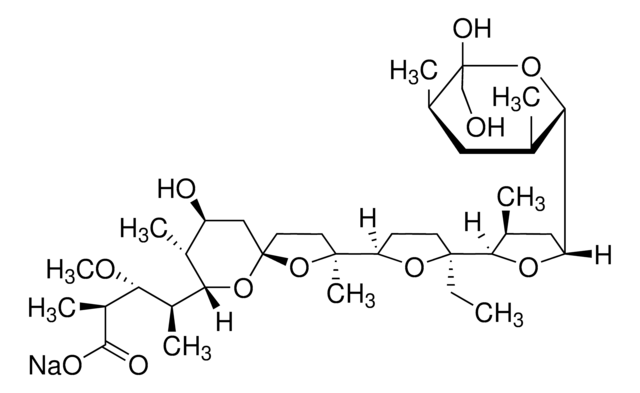
3B2-6379, Antibiotic K178, Antibiotic X464, Azalomycin M, HE331800, Helexin C, Polyetherin A, sodium;2-[(3S,6R)-6-[[(5R,6R,7R,9R)-2-[5-[(3S,5R)-5-[(2S,3S,5R,6R)-6-hydroxy-6-(hydroxymethyl)-3,5-dimethyloxan-2-yl]-3-methyloxolan-2-yl]-5-methyloxolan-2-yl]-7-methoxy-2,4,6-trimethyl-1,10-dioxaspiro[4.5]decan-9-yl]methyl]-3-methyloxan-2-yl]propanoate
About This Item
Polecane produkty
product name
Nigericin sodium salt Ready Made Solution, 5 mg/mL (DMSO:ethanol 1:1)
pochodzenie biologiczne
Streptomyces hygroscopicus
Poziom jakości
Postać
solution
stężenie
5 mg/mL (DMSO:ethanol 1:1)
spektrum działania antybiotyku
Gram-positive bacteria
Tryb działania
cell membrane | interferes
Warunki transportu
ambient
temp. przechowywania
2-8°C
InChI
1S/C40H68O11.Na/c1-21-11-12-28(46-33(21)26(6)36(42)43)17-29-18-30(45-10)27(7)40(48-29)25(5)19-38(9,51-40)32-13-14-37(8,49-32)35-23(3)16-31(47-35)34-22(2)15-24(4)39(44,20-41)50-34;/h21-35,41,44H,11-20H2,1-10H3,(H,42,43);/q;+1/p-1/t21-,22-,23-,24+,25?,26?,27+,28+,29+,30+,31+,32?,33?,34-,35?,37?,38?,39-,40+;/m0./s1
Klucz InChI
MOYOTUKECQMGHE-KKCUGXASSA-M
Powiązane kategorie
Działania biochem./fizjol.
Nigericin kills bacteria by facilitating the diffusion of ions across membranes.
Low concentration (0.5 μM) of Nigericin rapidly decreases pHi, causing stimulation of PG production 1.5- to 2-fold in cerebral microvascular endothelial cells and arresting of DNA synthesis in Erlich acites carcinoma cells. Treatment of Hela cells, after entry of poliovirus, with nigericin, prevents the inhibition of host protein synthesis by poliovirus. Nigericin is also widely used in studies of the consequences of changes in membrane potential in variable systems.
Uwaga dotycząca przygotowania
Przechowywanie i stabilność
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Flam. Liq. 3
Kod klasy składowania
3 - Flammable liquids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
78.8 °F
Temperatura zapłonu (°C)
26 °C
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej